Hepatitis D virus index
Index of pathogens
[Please click on the initial letter of the pathogen or simply scroll down the list!]
Vossen A
The microorganism and its clinical presentation
Hepatitis delta virus or hepatitis D virus (HDV) is a small single-stranded, circular RNA virus. It is a so-called defective virus, because it can only infect persons who are infected with hepatitis B virus (HBV). The HDV nucleocapsid is surrounded by the HBV surface antigen (HBsAg), which HDV uses to infect the host cells [Da 2019].
There are two ways a person can become infected with HDV. The HBV and HDV infection can take place simultaneously, the HBV-HDV co- infection, or HDV can be transmitted to a person with chronic hepatitis B, the so-called HDV super-infection. In immunocompetent adults an acute HBV-HDV co-infection leads to acute hepatitis which may be subclinical or mild but has an increased risk of acute liver failure, compared to acute HBV mono-infection. HBV-HDV viral clearance takes place in 95% of cases. A HDV superinfection in a HBV carrier can present as acute hepatitis, which may be misdiagnosed as acute hepatitis B or exacerbation of a chronic HBV. HDV superinfection leads to chronic infection in most cases, with an increased risk of progression to liver cirrhosis compared to chronic HBV mono-infection [Da 2019].
Complications
- Fulminant hepatic failure can occur in 1% of persons with HBV-HDV co-infection and in up to 5% after HDV super-infection [Da 2019].
Epidemiology
HDV is transmitted by exposure to blood or other infectious body fluids. Transmission rates are high among intravenous drug users and sexual transmission is also possible. Vertical transmission during delivery is uncommon. The incubation period of acute HDV is between 3 and 7 weeks.
About 4.5% of HBsAg-positive people are or have been infected with HDV as well, resulting in an estimated 12 million with evidence of HDV infection. The seroprevalence of HDV varies greatly per sub-population and country. In Europe, the estimated anti-HDV prevalence is 3% in HBsAg positive individuals in the general population, whereas it is 19.5% in individuals visiting a hepatology clinic [Stockdale 2020].
Diagnostic testing
The clinical, viral and serological course are depicted in figure 1.
Figure 1.
Techniques
- Detection of anti-HDV IgG or total Ig (IgM and IgG) is performed using (enzyme) immunoassays, such as Chemi-luminescent Immune Assay (CLIA) on automated analysers.
- Most of these assays detect total Ig, i.e. IgM and IgG, against HDV.
- After screening for anti-HDV, detection of HDV RNA is used to assess HDV replication.
- In acute HDV infection, serum HDV antigen appears early and is detectable for a short period. HDV Ag testing is not regularly performed.
Practical use of serology
Screening
Testing for immunity for HDV is not performed. In general, if a person is immune to HBV, they are also immune to HDV. (see also testing for immunity to HBV).
Suspected infection immunocompetent child or adult
- Diagnostic testing for HDV infection should be considered in all patients with chronic hepatitis B.
- In cases of clinical suspicion of co-infection or super-infection of HDV and hepatitis B, serology for both HDV and HBV should be performed, unless the person is known to have chronic HBV.
- The recommended test for HDV is anti-HDV IgG or anti-HDV total Ig.
- If the serology is positive, HDV RNA PCR can be performed.
Suspected infection immunocompromised child or adult
- In immunocompromised persons, HBsAg can be used for hepatitis B diagnostics and in general, anti-HDV can be used to screen for HDV. In severely immunocompromised individuals, HDV RNA PCR is recommended because of delayed or inadequate immune response.
Interpretation of serology
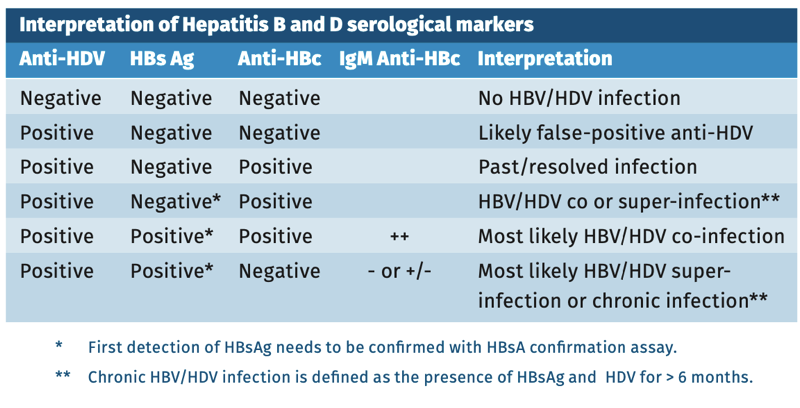
The interpretation of anti-HDV results in an immunocompetent person is depicted in table 1.
Table 1.
Sensitivity and specificity
Compared to reference laboratory results one of the studied commercial assays shows a high sensitivity (100%) and specificity (100%) in a small number of samples [Chow 2016]. The currently available commercial assay shows high concordance (97.5%) with the reference method [Rocco 2019].
pitfalls
- No specific pitfalls. See chapter “General pitfalls in serology”.
References
- Chow SK, Atienza EE, Cook L et al. Comparison of enzyme Immunoassays for detection of antibodies to hepatitis D virus in serum. Clin Vaccine Immunol. 2016 Aug 5;23(8):732-4.
- Da BL, Heller T, Koh C. Hepatitis D infection: from initial discovery to current investigational therapies. Gastroenterol Rep (Oxf). 2019 Jun 23;7(4):231-245.
- Rocco C, Bonavolta R, Vallefuoco L et al. Comparison of anti-hepatitis D virus (HDV) ETI-AB-DELTAK-2 assay and the novel LIAISON® XL MUREX anti-HDV assay in the diagnosis of HDV infection. Diagn Microbiol Infect Dis. 2019 Dec;95(4):114873.
- Stockdale AJ, Kreuels B, Henrion MYR et al. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J Hepatol. 2020 Sep;73(3):523-532.