Bordetella pertussis index
Index of pathogens
[Please click on the initial letter of the pathogen or simply scroll down the list!]
Petit P, Brandenburg A
The micro-organism and its clinical presentation
Bordetella pertussis is an encapsulated, non-motile, small, gram negative coccobacillus that grows aerobically with special growth requirements. Humans are its only host. Bordetella pertussis is transmitted by droplets and colonises the nasopharynx adhering specifically to ciliated cells. Subsequently the respiratory mucosa is primarily damaged by excretion of multiple exotoxins. An important virulence factor is pertussis toxin (PT) which is unique for Bordetella pertussis and is not produced by other Bordetella spp. PT probably disrupts host immune responses and is an essential factor in the induction of leucolymphocytosis [LCI 2018, Goldman 2009, Carbonetti 2015].
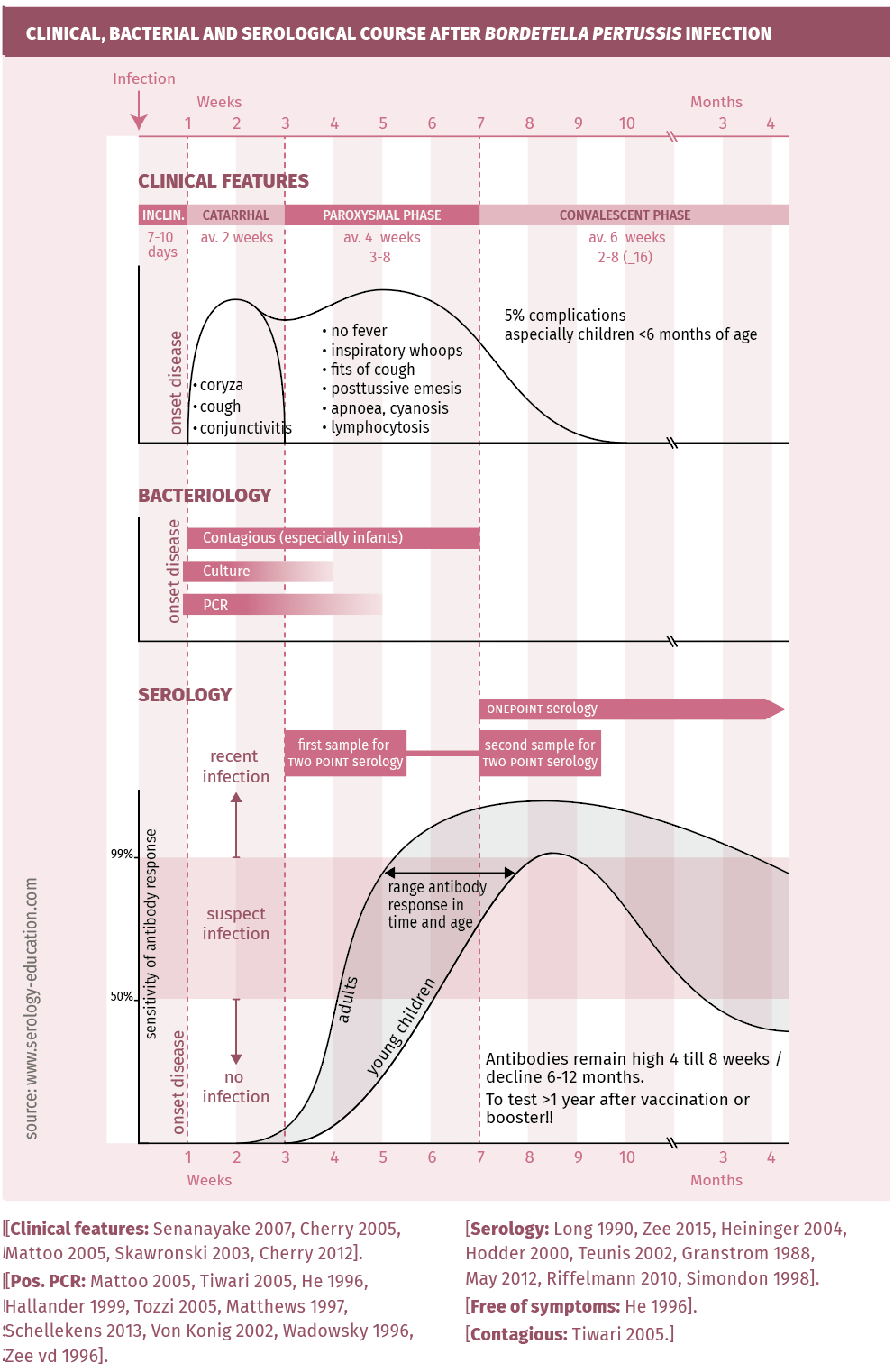
After an incubation time averaging 7-10 days (range 5-21 days), typical pertussis runs a three stage course (figure 1). It starts with nonspecific common cold-like symptoms and a slowly increasing cough lasting 1-2 weeks (catarrhal phase). Thereafter coughing becomes severe with copious mucus production and frequent expiratory paroxysms which often end with a profound inspiratory effort eliciting a sound called a “whoop”, often followed by post-tussive vomiting (paroxysmal phase, 3-8 weeks). However, in the youngest children and especially in premature newborns, typical symptoms may be absent; apnoeic and bradycardic episodes without whoops can be the dominant symptoms [Pasternak 1997]. Blood tests can show a very high leucocytosis, with a predominance of lymphocytes. In the third phase (convalescent phase 6-12 weeks), coughing slowly abates but can continue for many weeks to several months. While typical pertussis commonly manifests in immunenaïve individuals, symptoms of infection in vaccinated or previously infected individuals are atypical or mild and often not recognised as pertussis. “Pertussis-like” disease or cough can also be caused by B. parapertussis and other microorganisms such as Chlamydia pneumoniae, Mycoplasma pneumoniae and adenovirus. A clinical case definition aims to encompass all kinds of pertussis, using clinical, epidemiological and laboratory criteria [Senanayake 2007, Cherry 2005]: Coughing for at least two weeks combined with at least one of the following symptoms: inspiratory whoop, paroxysms, post-tussive emesis, apnoea and cyanosis (in the very young), or confirmed by one of the following laboratory results: culture, PCR or serology IgG-antiPT.
Complications
Five percent (5%) of individuals with classic pertussis develop complications. Complications are seen most frequently and are most serious in the very young (<6 months). More than half of children under one year old who develop pertussis require hospitalisation. Complications of otitis media or pneumonia can develop at any time during infection. Contrastingly, the most severe complications are seen during the paroxysmal phase: respiratory insufficiency and apnoea leading to convulsions and brain damage, subconjunctival haemorrhage, epistaxis, abdominal prolapses (hernia, rectum) and pneumothorax [Cherry 2005, Mattoo 2005, Skawronski 2003].
Epidemiology
Pertussis is an extremely contagious disease with a reproductive rate of 17 in an immune-naïve population. It is spread through coughing droplets containing B. pertussis. The milder the symptoms, the less contagious it is [Schellekens 2005]. Contagiousness is highest at onset of symptoms, decreasing throughout the paroxysmal phase, and disappears completely after 3-4 weeks (up to 6 weeks in infants), although complete resolution of coughing can take weeks [Heyman 2008, Long 1990, He 1996, Mink 1992, Tiwari 2005].) Bordetella pertussis infections are re-emerging, causing endemic disease worldwide with seasonal summer peaks and epidemic waves every 2-4 years, even in highly vaccinated populations. This development is probably caused by vaccine changes (with more rapidly decreasing antibodies) and by molecular changes within strains of B. pertussis itself [Pasternak 1997, Zee 2015, Mooi 2009]. From the start of the vaccination era, the majority of pregnant women have low levels of antiPT-IgG; these antibodies, probably not protective, rapidly decrease after the birth of the child (absent by 2 months of age), which leaves these infants vulnerable to infection. Due to the infant’s immature immune system and interference from maternal antibodies, vaccination in infants <3 months does not provide a solution to this problem. However, acellular vaccines administered during pregnancy possibly provide a solution [Switzer 2019]. The role of genetic modification in reducing protection of the acellular Pertussis Vaccine as yet remains unclear.
The level of antibodies (and protection) after vaccination is not longlasting (mostly a decline within 1–3 years), and shows individual and age differences.
Waning of antibody protection against reinfection and the circulation of Bordetella pertussis variants depleted of antigens used for vaccineinduced antibodyproduction in individuals favour the increase in pertussis disease [Esposito 2019]. Symptoms vary depending on the degree of residual immunity, ranging from no symptoms to mild or chronic cough, or rarely to typical pertussis symptoms; these infections are rarely recognised by physicians [De Greeff 2010, Althouse 2015, Cromer 1993, Ward 2005, Long 1990, Zhang 2014]. Over recent years, increasing awareness and more sensitive laboratory techniques for diagnosis have increased the number of pertussis notifications, showing a population-based infection rate of around 6% per year [Cromer 1993, de Greeff 2010]. The reservoir of transmission is children >12 years and adults (spreading to infants too young to be vaccinated [de Greeff 2010, Althouse 2015, Ward 2005, Long 1990, Zhang 2014]. As deduced from serosurveys using IgG-Pertussis toxin assays, the incidence of B. pertussis infection in individuals >9 years increased from 4.0% per year in 1995-96 to 9.3% per year in 2006-07 [De Greeff 2010, Cromer 1993, Melker 2000]. In the prevaccine era, pertussis was responsible for more infant deaths than scarlet fever, diphtheria, meningitis and poliomyelitis combined [Pasternak 1997]. Nowadays there still is an estimated worldwide incidence of 24.1 million infections in children aged <5 years with 160,700 deaths [Yeung 2017]. In the Netherlands, annually one child <2 year dies from pertussis [LCI 2018].
Diagnostic testing
The clinical, bacterial and serological course are depicted in figure 1.
figure 1.
Techniques
A summary of the techniques used to diagnose pertussis can be found in references: LCI 2018, Cherry 2005, Zee 2015, Hallander 1999, Tozzi 2005, Matthews 1997, Horby 2005.
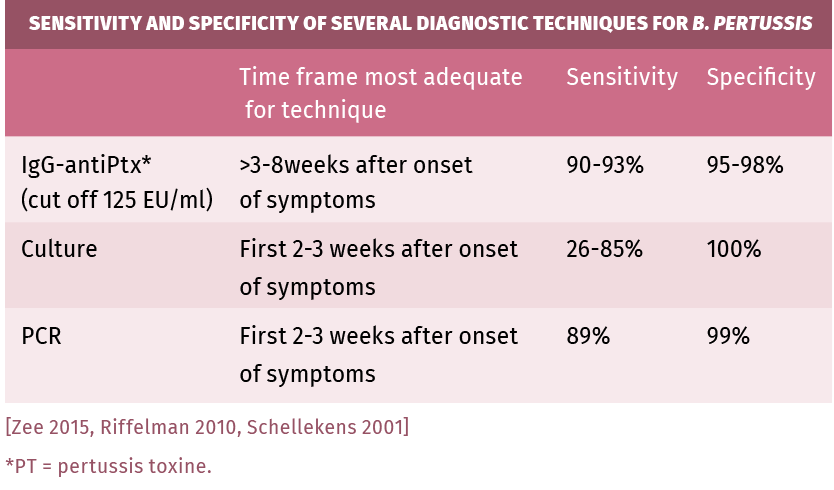
Culture: Bordetella pertussis needs specific media and growth conditions for transport and culture. Even then, culture is only positive in 50% of clinical cases. Sensitivity is highest early in the catarrhal phase and declines through the paroxysmal phase (3 weeks after infection). Cultures are necessary for genotyping and antimicrobial susceptibility testing. In very young children, cultures can remain positive for longer periods of time [Lee 2018, Zee 2015].
PCR is the first choice for diagnosis in infants and children, provided adequately specific primers are used. It is a reliable technique to detect Bordetella pertussis infections during the early (catarrhal) phase of infection [Melker 2000, Wadowsky 1996, Zee 1996]. PCR can detect infection from its onset until 4 weeks after onset of symptoms. Its sensitivity is higher than culturing, however it does not differentiate between live and dead bacteria. Like in culturing, sensitivity quickly declines in the paroxysmal phase [Lee 2018, Zee 2015].
Serology: ELISAs for IgG using purified PT (Pertussis toxin) as antigen and calibrated with International Reference preparations are the preferred tests for serology. Results are expressed in the “CBR-EU/ ml” or “IU/ml” allowing for a uniform diagnostic cut-off [Long 1990, Mink 1992, Lynn 1996, Xing 2009, Heininger 2004, Hodder 2000, Teunis 2002, Granstrom 1988, De Greeff 2012, Schellekens 2013]. Since production of pertussis toxin is exclusive to Bordetella pertussis, no cross-reactions should occur when PT antigen-based ELISAs are used. When using other or mixed antigens , cross-reactions can occur with other Bordetella species and also with Haemophilus sp., Mycoplasma pneumoniae and E. coli [Tondella 2007].
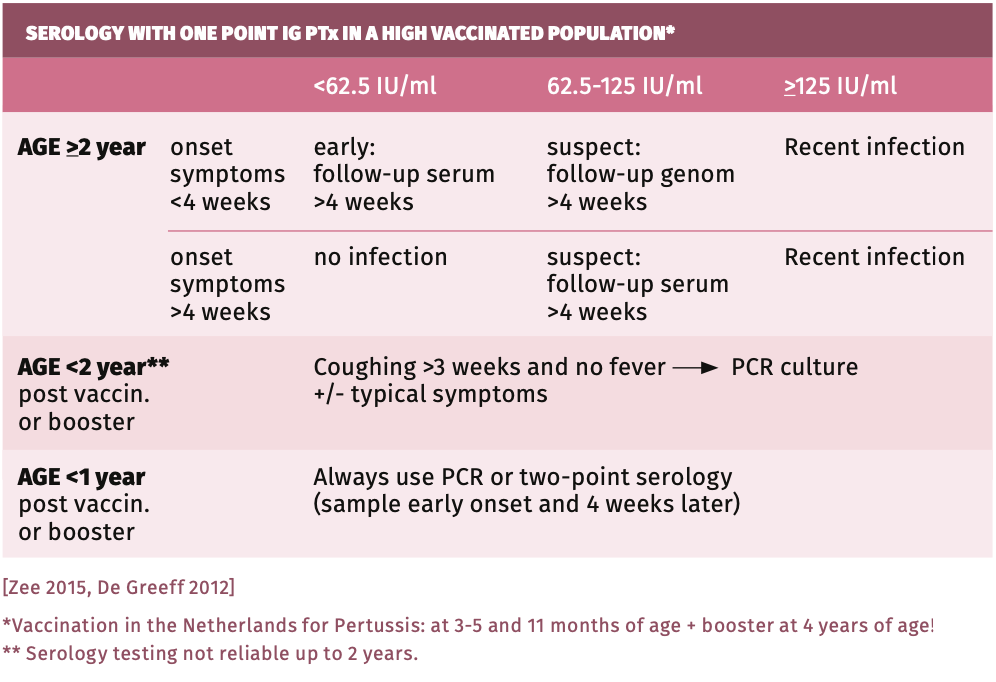
The total titer of IgG in blood is high in children and decreases with age. This should be taken into account when interpreting Bordetella pertussis serology, be it in suspected infection or post-vaccination. High cut-offs for IgG-PT were best for reliable interpretation of ELISA results [Fumimoto 2019, Jogi 2020, Lee 2018, Markey 2019, Pawlowski 2017, Schellekens 2013, Schellekens 2001, Twillert 2016]. See table 1. There is definite advice against use of tests other than EIAs, such as microagglutination, CBR, indirect immuno-fluorescence and immunoblot due to their low sensitivity. There is no place for IgM tests [Zee 2015, Pawloski 2017, Fumimoto 2019].
IgA serology Bordetella pertussis: IgA could be used to differentiate positive serology due to vaccination and infection, since vaccination does not invoke an IgA response [May 2017, Nagel 1983, Poynten 2002, Schellekens 2013]. B. pertussis IgA tests, however, have several drawbacks: they yield a high number of false-positives (unless the more specific IgA-antiPT is used). In addition, in young age, IgA is often not yet produced (<62%) while at an older age IgA may be positive due to background prevalence. Because of these drawbacks, unless age-dependent cut-offs are used, IgA tests should not be used. Although some recommend using IgA [Subissi 2020, Jogi 2020, May 2017], only the reliability of IgG-antiPT (with cut-off 125 IU/ml, specificity 99%) has been confirmed by CDC and other quality assessments [Lee 2018, Pawlowski 2017, Markey 2019, Fumimoto 2019]).
When still in doubt, a second serum should be tested for IgG-antiPT to increase sensitivity.
PRACTICAL USE OF SEROLOGY
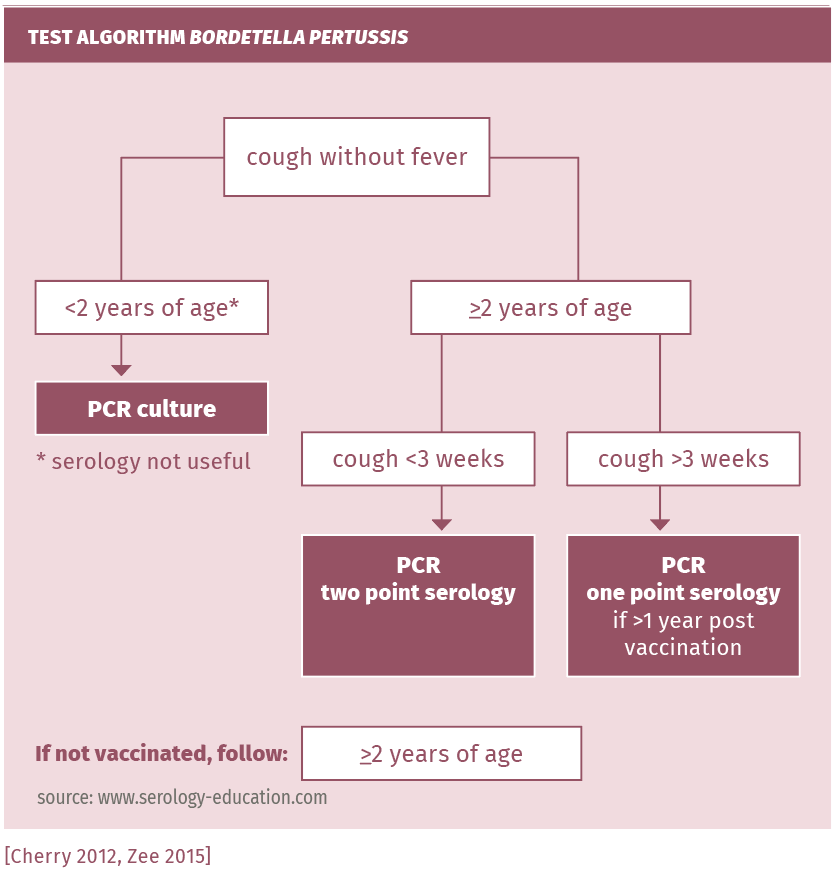
See figure 2.
figure 2.
Screening
Screening for seroprevalence is done with IgG-PT ELISA.
Suspected infection in immunocompetent patients
Perform one point IgG-antiPT (with titer cut-off >125 IU/ml for positive) in the following cases: cough for more than 3 weeks, whether in attacks or not, and in contacts of any age with airway complaints in the direct vicinity of a patient with whooping cough [Schellekens 2013, Schellekens 2001, Lee 2018, Pawlowski 2017]. If found negative or low in symptomatic patients, the test should be repeated after 2-3 weeks because of the slow immunoresponse (the antibody response can take 6-8 weeks to develop). A second serum sample obtained 2 to 3 weeks later should be tested. In the second of the paired sera, a threefold rise of the antiPT-IgG up to >20 IU/ml proves an actual infection by Bordetella pertussis (specificity almost 100%). If recently vaccinated or boostered, PCR testing on a nasopharyngeal swab should be performed instead (see table 2).
Suspected infection in children <2 years
Pertussis should be considered when respiratory symptoms (including cough) persist beyond 10-14 days. The dynamic phase of the immune response to Bordetella pertussis is often delayed until 2 to 4 weeks or even 6 to 8 weeks (in the youngest children) after onset of symptoms. A negative result in the first serum, therefore, does not exclude the diagnosis of pertussis.
Suspected infection in immunocompromised patients
IgG-PT may be used; however, if antibodies are absent, PCR should be performed.
Vaccination
A single point high IgG-PT titer within up to 12 months after pertussis vaccination or boostering does not prove a recent infection. Depending on the vaccination schedule used, children >3 months and recently vaccinated or boostered patients (in Netherlands given at 3-5 months, 11 months and 4 years of age) can be reliably diagnosed using preferably PCR or culture, or paired sera (two point serology)! In the Netherlands, for children >2 years and adults, PCR or one point serology if >1 year post vaccination and two point serology within 1 year post vaccination/boostering are preferred.
Confirmation
Serological confirmation of recent infection 1-3 years after vaccination or boostering is performed by the RIVM using a combined IgG/IgA test validated with age-dependent cut-offs [Poynten 2002, Riffelmann 2010, Schellekens 2015].
interpretation of serology
Table 1.
Sensitivity and specificity
Table 2.
pitfalls
- Antibody response may take up to 4 weeks in older children and adults, and up to 8 weeks in children <1 year (see figure 1).
- IgA tests have a low sensitivity in children and a high false positive rate because of common use of too low a cut-off value in all ages.
- Vaccination(including the acellular vaccins) induces higher levels of antibodies against Ptx and complicates the serodiagnosis of pertussis in the first 6-12 months after vaccination.
- Check the antigen used in the test: tests based on other antigens than Ptx are not specific and show many cross-reactions.
- Check whether commercial IgG-Ptx EIA has been calibrated with the international WHO standard serum c.q. express results in “IU/ml”.
- Also see general chapter "Pitfalls".
References
- Althouse BM, Scarpino SV. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med 2015;13:146.
- Carbonetti NH. Contribution of pertussis toxin to the pathogenesis of pertussis disease. FEMS Pathogens and diseases 2015;73(8):ftv073.
- Cherry JD, Grimpre lE, Guiso N et al. Defining pertussis epidemiology: clinical, microbiological and serologic perspectives. Ped Inf Dis J 2005;24(suppl.5): S25-S34.
- Cherry JD, Tan T, Wirsing von Konig CH et al. Clinical definitions of Pertussis: Summary of a global pertussis initiative roundtable meeting February 2011. Clin.Inf Dis 2012;54:1756-1764
- Cromer BA, Goydos J, Hackell J et al. Unrecognized pertussis infection in adolescents. Am J Dis Child 1993;147:575-577.
- De Greeff SC, Mooi FR, Westerhof A et al. Pertussis disease burden in the household: how to protect young infants. Clin Inf Dis 2010;50:1339-45.
- De Greeff SC, Teunis P, de Melker HE et al. Two-component cluster analysis of a large serodiagnostic database for specificity of increases of IgG antibodies single serum samples. Clin Vaccine Immunol 2012;19:145-56.
- De Greeff SC, deMelker HE, van Gageldonk PG et al. Seroprevalence of pertussis in The Netherlands: evidence for increased circulation of Bordetella pertussis. PLoSOne 2010;5:e14183.
- Esposito S, Stefanelli P, Fry N et al. Pertussis prevention: reasons for resurgence, and differences in the current acellular pertussis vaccines. Front.Immunol. 2019;10 art 1344.
- Fumimoto R, Otsuka N, Sunagawa T et al. Age-related differences in antibody avidities to pertussis toxin and filamentous haemagglutinin in a healthy Japanese population. Vaccin 2019;37(18):2463-2469.
- Goldman E, Green LH: Practical Handbook of Microbiology, 2nd ed.2009 chapter38: The genus Bordetella by Stenson TH, Peppler MS.
- Granstrom G, Wretlind B, Salenstedt CR et al. Evaluation of serologic assays for diagnosis of whooping cough. J Clin Microbiol 1988;26:1818-1823.
- Hallander HO. Microbiogical and serological diagnosis of Pertussis. Clin Inf Dis 1999;28(suppl2):S99-106.
- He Q, Schmidt Schlapfer G, Just M et al. Impact of polymerase chain reaction on clinical pertussis research: Finnish and Swiss experiences.
- J Inf Dis 1996;174(6):1288-95.
- Heininger U, Cherry JD, Stehr K: Serologic response and antibody-titer decay in adults with pertussis. Clin Infect Dis 2004;38:591-594.
- Heymann D.(2008). Control of communicable diseases manual. 19th edition. Washington, American Public Health Association.
- Hodder SL, Cherry JD, Mortimer EA et al. Antibody responses to Bordetella pertussis antigens and clinical correlations in elderly community residents. Clin Inf Dis 2000;31:7-14.
- Horby P, Macintyre CR, McIntyre PB et al. A boarding school outbreak of pertussis in adolescents: value of laboratory diagnostic methods. Epid. Infect 2005;133:229-236.
- Jogi P, Soeong H, Oona M et al. Dynamics of pertussis toxin IgG after symptomatic pertussis in children and adults. Vaccin 2020;38(16):3196200.
- LCI richtlijn 2018: Kinkhoest, RIVM.
- Lee A, Cassidy PK, Pawloski LC et al. Clinical evaluation and validation of laboratory methods for the diagnosis of Bordetella pertussis infection: culture, polymerase chain-reaction (PCR) and anti-pertussis toxin IgG serology (IgG-PT). PlosSone 2018;13(4):e0195979.
- Long SS, Welkon CJ, Clark JI. Widespread silent transmission of Pertussis in families: antibody correlates of infection and symptomatology. J Inf Dis 1990;161:480-486.
- Lynn F, Reed GF, Meade BD. Collaborative study for the evaluation of Enzymelinked immunosorbent assays used to measure human antibodies to Bordetella pertussis. Clin Diagn Lab Immunol 1996;3:689-700.
- Markey K, Douglas-Baredsley A, Asokanathan C et al. Improved serological diagnosis of pertussis by external by external quality assessment. J Med Microb 2019;68(5):741-747.
- Matthews R. The diagnosis of pertussis infection. PHLS Microbiology Digest 1997;14(2).
- Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Micr Rev 2005;18(2):326-827.
- May ML, Doi SA, King D et al. Prospective evaluation of an Australian pertussis toxin IgG and IgA enzyme immunoassay. Clin Vaccine Immunol 2012;19:190197.
- May ML, Evans J, Holgate T et al. Pertussis toxin IgA testing over-diagnoses recent pertussis infection. Pathology 2017;49(7):770-775.
- Melker de HE, Versteegh FG, Conyn-VanSpaendonck MAE et al. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J Clin Microbiol 2000;38:800-806.
- Mink CM, Cherry JD, Christenson P et al. A search for Bordetella pertussis infection in university students. Clin Inf Dis 1992;14:464-471.
- Mooi FR, vLooI HM et al. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emergent Infect Dis 2009;15(8):1206-1213.
- NageI J, Poot-Scholtens EJ. Serum IgA antibody to Bordetella pertussis as an indicator of infection. J Med Microbiol 1983;16:417-426.
- Pasternack MS. Pertussis in the 1990s: Diagnosis, treatment, and prevention. In Current Clinical Topics in Infectious Diseases by Remington JS & Swartz MN 1997 Nr.17:24-36.
- Pawlowski LC, Brian D, Martin MD et al. Evaluation of commercial assays for single point diagnosis of pertussis in the US. J of Ped Inf Dis Society 2017;6(3):e15-e21.
- Poynten M, Hanlon M, Irwig L et al. Serological diagnosis of pertussis: evaluation of IgA against whole cell and specific Bordetella pertussis antigens as markers of recent infection. Epidemiol Inf 2002;128:161-67.
- Qi Zhang, Zundong Yin, Yixing Li et al. Prevalence of asymptomatic Bordetella pertussis and Bordetella parapertussis infections among school children in China as determined by pooled real-time PCR: A cross-sectional study. Scandin J of Inf Dis 2014;46(4):280-287.
- Riffelmann M, Thiel K, Schmetz J et al. Performance of commercial enzymelinked immunosorbent assays for the detection of antibodies to Bordetella pertussis. J Clin Micr 2010;4459-4463.
- Schellekens J, Boshuizen H, Verbakel J et al. Serodiagnosis of pertussis with commercial Elisas. ICAAC, Chicago USA, 2001, abstractbook page 163,abstractnumberD-143.
- Schellekens J, Wirsing von Konig CH, Gardner P et al. Pertussis sources of infection and routes of transmission in the vaccination era. Pediatr. Inf Dis J 2005;24:S19-24.
- Schellekens JFP. Kinkhoestserologie. Ned Tijdschr Med Microbiol 2013;21(3): 107-112.
- Simondon F, Iteman I, Preziosi MP et al. Evaluation of an immunoglobulin G enszyme-linked immunosorbent assay for pertussis toxin and filamentous hemagglutinin in diagnosis of pertussis in Senegal. Clin Diagn.Lab. Immunol.1998;5(2):130-134
- Skawronski Danuta M, Buxton JA, Hestrin M et al. Carotid artery dissection as a possible severe complication of pertussis in an adult: Clinical case report and review. Clin Inf Dis 2003;36(1):1-4.
- Senanayake S. Clinical cases in Infectious Diseases: a public health approach. 2007;Case16 Pertussis267-287.
- Subissi L, Rodeghiero C, Martini H et al. Assessment of IgA anti-PT and IgG anti-ACT reflex testing to improve Bordetella pertussis serodiagnosis in recently vaccinated subjects. Clin Micro and Inf 2020;26(5):645e1-645e8.
- Switzer C, D’Heilly C, Macina D. Immunological and clinical benefits of maternal immunization against pertussis: a systematic review. Inf Dis Ther 2019;8:499-541.
- Teunis PFM, Heijden vd OG, de Melker HE et al. Kinetics of the IgG antibody reponse to pertussis toxin after infection with B.pertussis. Epidem Infect 2002;129:479-489.
- Tiwari T, Murphy TV, Moran J. Recommended antimicrobial agents for the treatment and post-exposure prophylaxis of pertussis. CDC guidelines 2005. MMWR 54(RR-14):1-16.
- Tondella ML, et al. International Bordetella pertussis assay standardization and harmonization meeting report. CDC and Prev, Atlanta. Vaccin 2007;27:803-814.
- Tozzi AE, Celentano LP, Ciofi AML, Salmaso S. Diagnosis and management of pertussis. Can Med Ass J 2005;172(4):509-515.
- Von Konig CH, Halperin S, Riffelmann M et al. Pertussis of adults and infants. Lancet Infect Dis 2002;2(12):744-750.
- Wadowsky RM, Michaels RH, Libert T et al. Multiplex-PCR-based assay for detection of Bordetella pertussis in nasopharyngeal swab specimens. J Clin Micr 1996;34:2645-49.
- Ward JL, Cherry JD, Chang SJ et al. Efficacy of an acellular pertussis vaccine among adolescents and adults. N Eng J Med 2005;353:1555-1563.
- Xing D, Wirsing von Konig CH, Newland P et al. Characterization of reference materials for human antiserum to pertussis antigens by an international collaborative study. Clin Vacc Immunol 2009;16:303-311.
- Yeung KHT, Duclos P, Nelson EAS et al. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Inf Disd 2017;17(9):974-80.
- Zee vander A, Agterberg C, Peeters M et al. A clinical validation of Bordetella pertussis and Bordetella parapertussis polymerase chain reaction: comparison with culture and serology using samples from patients with suspected whooping cough from highly immunized population. J Inf Dis 1996;174:89-96.
- Zee vander A, Schellekens JFP, Mooi FR. Laboratory diagnosis of Pertussis. Clin Microb Rev 2015;28(4):1005-1026.
Keywords: Bordetella pertussis, Whooping cough