Measles virus index
Index of pathogens
[Please click on the initial letter of the pathogen or simply scroll down the list!]
Bodewes r, Voordouw B, Petit p
The microorganism and its clinical presentation
Measles is a highly contagious respiratory viral infection caused by the measles virus, an enveloped, single stranded RNA-virus, member of the Morbillivirus genus in the family of Paramyxoviridae [Moss 2017, ICTV 2020]. 24 measles virus genotypes have been recognized, genotypes most frequently reported in the EU region in recent years are B3 and D8 [Brown 2019].
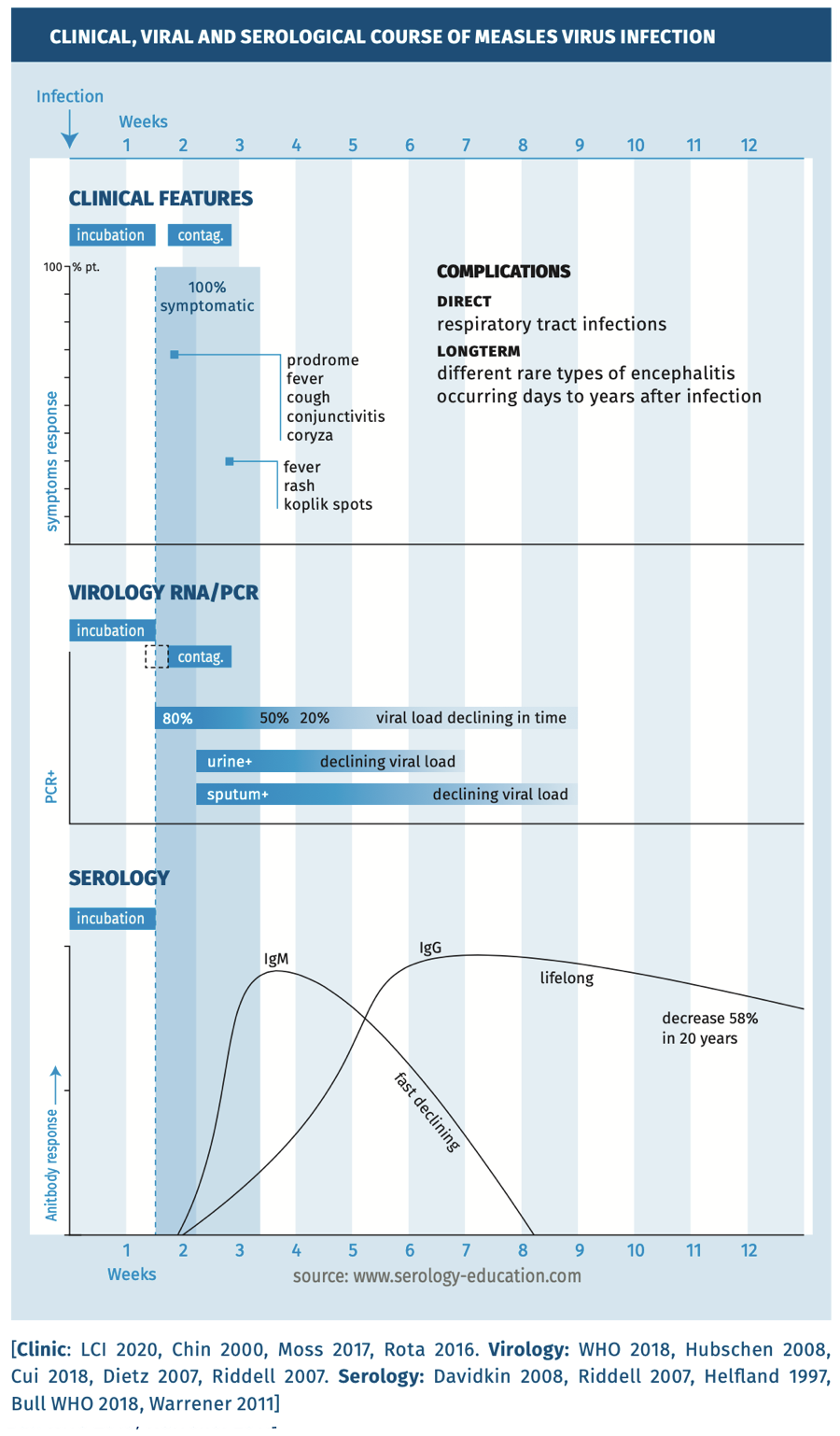
Incubation time from infection to onset of initial symptoms is approximately 10 days (7-18) [Moss 2017]. Initial prodromal symptoms usually include a high fever (often >40°C), Koplik spots, malaise, loss of appetite, red eyes, runny nose, and sometimes cough (typical 3 C’s: cough, conjunctivitis, coryza). Koplik spots are mouth-clustered white spots in the buccal mucosa of the mouth and are considered pathognomonic for measles. They usually appear 2–3 days prior to the occurrence of the characteristic rash that may last 3–7 days [Rota 2016]. The rash is typically maculopapular and erythematous and covers much of the body [Naim 2015]. Leucopenia is common in this stage. The infectious period starts shortly before the prodromal period, around 4 days before and lasts till 4 days after onset of rash [Rota 2016]. Live attenuated measles vaccine is used and may produce in 5-15% a mild measles-like or inapparent non-communicable disease!
Complications
Complications resulting from viral replication or bacterial superinfection occur especially in malnourished children. Measles infections cause a transient immune suppression, resulting in increased susceptibility to opportunistic infections [Mina 2015, Mina 2019, Gadroen 2018, Laksono 2018].
Particularly at risk are those too young to be immunized or older patients and individuals with vitamin A deficiency. A substantial number of measles infections requires hospitalization, mainly for respiratory superinfections, with 22% reported in France to 30% in South Africa [Kabra 2013]. Case-fatality rate is between 0,5-6% [Rota 2016, Cairns 2010].
In approximately 1 in 1000 patients acute or late encephalitis or post- measles encephalopathy occurs within days-weeks-months following infection. Another rare complication, the subacute sclerosing panencephalitis (SSPE), is a delayed complication occurring 5-10 years after acute illness and reported in 1:10.000 to 1:100.000 patients, mainly in children acquiring measles before the age of 2 years. After progressive deterioration, SSPE results in death [Moss 2017].
Epidemiology
The epidemiology is largely determined by the respiratory mode of transmission through droplets or direct contact with nasal or throat secretions of infected persons; less commonly by airborne spread or through secretions of nose and throat [Moss 2017, WHO 2020]. Estimated basic reproductive rate of 12-18 [LCI 2020]. The estimated burden of infection for measles depends upon the vaccination coverage. In temperate climates annual outbreaks of measles typically occur in winter and early spring. In tropical regions outbreaks have a more variable seasonal pattern, and in regions with high birth rates, highly irregular large measles outbreaks may occur. Measles vaccination coverage also reflects the main age of infection, with lower age in low vaccination coverage settings [Moss 2017, Rota 2016]. Neonates are protected by maternal antibodies only in the first months [Rota 2016, Waaijenborg 2013].
Diagnostic testing
The clinical, viral and serological course are depicted in figure 1.
Figure 1.
Techniques
- Measles RNA-PCR can be used on various clinical specimens, such as throat swab, oral fluid or urine sample. Samples for PCR can be collected within 3 days after the onset of clinical signs (exanthema) to 5 weeks for urine or to 7 weeks for sputum [WHO 2018].
- Serology: detection of anti-measles IgM antibodies can be performed using an EIA test to prove a recent/acute infection on a blood sample taken from 4 days after onset of rash. A laboratory diagnosis can also be based on seroconversion or a more than four-fold titer rise in measles virus- specific IgG in paired sera (at least two weeks in between) or collected during the acute and convalescent phase of the disease [WHO 2018, Hubschen 2017, Ma 2019, Helfland 1997]. For prevalence studies IgG EIA’s are the first choice.
- Avidity testing can be performed using anti-measles IgG antibodies to confirm the duration of time after exposure [Paunio 2000, Mercader 2012]*.
* Avidity testing is used in breakthrough infections (often without IgM response) or if test results are inconclusive and only one serum Is available.
Practical use of serology
Screening
Test EIA-IgG measles antibody in case contact of investigations or confirmation of specificity antibodies in a patient with a positive IgM antibody test. However, the current EIA assays have suboptimal sensitivity and therefore have limitations for determine protective antibodies against measles vaccinated individuals [Dorigo-Zetsma 2015].
Suspected infection in immunocompetent child or adult
Suspected infection is tested for the presence of anti-measles IgM antibodies using a measles-specific capture IgM EIA. In breakthrough infections or suspected measles infection in a person with a recent history of travel in a measles endemic region an IgM must be confirmed with a PCR from throat swabs, oral fluid or urine [Bolotin 2017, Cui 2018, Helfland 1998]. However, in infected individuals - previously vaccinated - there is often no IgM response. In this situation the practical first choice to prove infection is PCR on a throat, saliva or urine sample. An IgM test can be performed only if a PCR sample (taken late after onset) is negative.
Suspected infection in immunocompromised child or adult
Patients with measles exposure may not develop clinical signs of measles (fever, skin rash, cough, coryza or conjunctivitis) while measles infection can cause severe and even fatal disease and may not have a detectable IgM response. These patients should therefore be tested with PCR.
INTERPRETATION of SEROLOGY
In symptomatic but vaccinated patients a single serum sample can not provide definite proof of an infection. For diagnostic purpose always collect sample(s) for PCR within 1 week of onset symptoms (see table 1) or use paired serum samples whatever the result of the first sample to prove a fourfold/significant titre rise.
TABLE 1. Interpretation serology in an immunocompetent person*
SENSITIVITY AND SPECIFICITY
Sensitivity of serology is best in the convalescence phase 6-14 days after the onset of symptoms (see table 2).
TABLE 2.
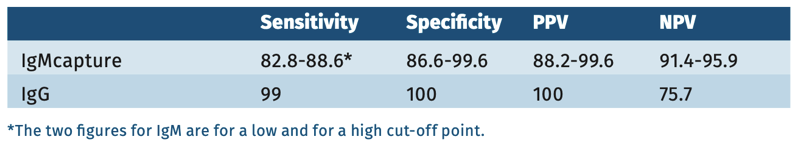
According to WHO evaluation, the IgM anti-measles antibody testing had a sensitivity of 75-98.1% and a specificity of 86.6-99.5%, with possible false positives in Parvovirus IgM B19 positive samples. The IgM capture test performed best [Hiebert 2021].
Pitfalls
- In persons previously vaccinated for measles, breakthrough infections can occur, often with relatively mild symptoms. In these cases, measles virus-specific IgM can remain below the detection levels of the available EIA, while measles virus-specific IgG antibodies are detected. In persons suspected of having measles infection, who have been previously vaccinated, either collection of PCR samples or paired serum samples, to detect significant anti-measles IgG titer increase, is recommended [Hahné 2016, Sundell 2019].
- Vaccination with the MMR vaccine can also result in a measles virusspecific IgM response [Helfland 1998] and measles-like symptoms within 2 weeks after vaccination and this does not have to be tested!
- Differentiation between vaccine-derived virus and wild type virus can be achieved through specific measles PCRs and through PCR amplification and subsequent sequencing in order to determine the measles virus genotype [Roy 2017, Tran 2018].
References
- Bolotin S, Lim G, Dang V et al. The utility of measles and rubella IgM serology in an elimination setting, Ontario, Canada, 2009-2014. PLoS One 2017;12(8): p. e0181172.
- Brown KE, Rota PA, Goodson JL et al. Genetic Characterization of Measles and Rubella Viruses Detected Through Global Measles and Rubella Elimination Surveillance, 2016-2018. MMWR Morb Mortal Wkly Rep 2019;68(26): p. 587591.
- Cairns KL, Nandy R, Grais RF. Challenges in measuring measles case fatality ratios in settings without vital registration. Emerg Themes Epidemiol 2010;7(1): p. 4.
- Chin J. Control of communicable Diseases manual. 17th edition 2000;Chapter Measles pg 330-335.
- Cui A, Mao N, Wang H et al. Importance of real-time RT-PCR to supplement the laboratory diagnosis zzzin the measles elimination program in China. PLoS One 2018;13(11): p. e0208161.
- Davidkin I, Jokinen S, Broman M et al. Persistence of measles, mumps and rubella antibodies in an MMR-vaccinated cohort: a 20 year follow-up. J Infect Dis 2008;197:950-56.
- Dietz V, Rota J, Izurieta H et al. The laoratory confirmation of suspected measles cases in settings of low measles transmission: conclusion from the experience in the Americas. Bull. WHO 2004;82:852-857.
- Dorigo-Zetsma JW, Leverstein-Van Hall MA, Vreeswijk J et al. Immune status of Health care workers to measles virus: evaluation of protective titers in four measles IgG EIA’s. J Clin Virol 2015;69:214-218.
- Gadroen K, Dodd CN, Masclee GM et al. Impact and longevity of measlesassociated immune suppression: a matched cohort study using data from the THIN general practice database in the UK. BMJ Open 2018;8(11): p. e021465.
- Hahné SJM, Lochlainn LMN, Burgel van ND et al. Measles Outbreak Among Previously Immunized Healthcare Workers, the Netherlands, 2014. J Infect Dis 2016;214(12): p. 1980-1986.
- Haywood B, Patel M, Hurday S et al. Comparison of automated chemiluminescence immunoassays with capture enzyme immunoassays for the detection of measles and mumps IgM antibodies in serum. J Virol Methods 2014;196: p. 15-7.
- Helfland RF, Heath JL, Anderson LJ et al. Diagnosis of measles with an IgM capture EIA: the optimal timing of specimen collection after rash onset. J Infect Dis 1997;175(1): p. 195-9.
- Helfland RF, Kim DK, Gary jr HE et al. Nonclassic measles infections in an immune population exposed to measles during a college bus trip. J Med Virol 1998;56(4): p. 33741.
- Hiebert J, Zuback V, Charlton CL et al. Evaluation of diagnostic accuracy of eight commercial assays for the detection of measles virus specific IgM antibodies. J Clin Micr 2021;doi:10.1128.
- Hubschen JM, Kremer JR, De Landtsheer S et al. A multiplex Taqman PCR assay for the detection of measles and rubella virus. J Virol Methods 2008;149:2465.
- Hubschen JM, Bork SM, Brown KE et al. Challenges of measles and rubella laboratory diagnostic in the era of elimination. Clin Microbiol Infect, 2017. 23(8): p. 511515.
- ICTV. Virus Taxonomy: 2019 Release. 2020; Available from: https://talk. ictvonline.org/taxonomy/.
- Kabra SK and Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev 2013(8):p.CD001477.
- Laksono BM, Vries deRD, Verburgh RJ et al. Studies into the mechanism of measles-associated immune suppression during a measles outbreak in the Netherlands. Nat Commun 2018;9(1): p. 4944.
- LCI: richtlijn Measles 2020.
- Ma R. Lu L, Suo L et al. Evaluation of the adequacy of measles laboratory diagnostic tests in the era of accelerating measles elimination in Beijing, China. Vaccine 2019;37(29): p. 3804-3809.
- Mercader S, PGarcia P, and Bellini WJ. Measles virus IgG avidity assay for use in classification of measles vaccine failure in measles elimination settings.
- Clin Vaccine Immunol 2012;19(11): p. 1810-7.
- Mina MJ, Metcalf CJ, Swart RL et al. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science 2015;348(6235): p. 694-9.
- Mina MJ, Kula T, Leng Y et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science 2019;366(6465): p. 599606.
- Moss WJ. Measles. Lancet 2017;390(10111): p. 2490-2502.
- Naim HY. Measles virus. Hum Vaccin Immunother 2015;11(1): p. 21-6.
- Neumann PD, Weber JM, Jessamine AG et al. Comparison of measles antihemolysin test, Enzyme-Linked-Immunosorbent assay, and Haemaggluti nation Inhibition test with Neutralization test for determination of immune status. J Clin Micr 1985;22(2):296-298.
- de Ory F, Minguito T, Balfagon P et al. Comparison of chemiluminescent immunoassay and ELISA for measles IgG and IgM. APMIS 2015;123(8): p. 648-51.
- Panda BK, Mishra S, Awofeso N. Socio-demographic correlates of first dose of measles (MCV1) vaccination coverage in India. BMC Public Health 2020;20(1): p. 1221.
- Paunio M, Hedman K, Davidkin I et al. Secondary measles vaccine failures identified by measurement of IgG avidity: high occurrence among teenagers vaccinated at a young age. Epidemiol Infect 2000;124(2): p. 263-71.
- Ratnam S, Tipples G, Head C et al. Performance of indirect immunoglobuline M(IgM) for laboratory diagnosis of measles. Journal of Clin Mic. 2010;(38):99104
- Riddell MA, Moss WJ, Hauer D et al. Slow clearance of measles virus RNA after acute infection. J Clin Virol 2007;39:312-17
- Rota PA, Moss WJ, Takeda M et al. Measles. Nat Rev Dis Primers 2016;2: p. 16049.
- Roy F, Mendoza L, Hiebert J et al. Rapid Identification of Measles Virus Vaccine Genotype by Real-Time PCR. J Clin Microbiol 2017;55(3): p. 735-743.
- Sundell N, Dotevall L, Sansone M. Measles outbreak in Gothenburg urban area, Sweden, 2017 to 2018: low viral load in breakthrough infections. Euro Surveill 2019;24(17).
- Tran T, Kostecki R, Catton M et al. Utility of a Stressed Single Nucleotide Polymorphism (SNP) Real-Time PCR Assay for Rapid Identification of Measles Vaccine Strains in Patient Samples. J Clin Microbiol 2018;56(8).
- Waaijenborg S, Hahne SJM, Mollema L et al. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. J Infect Dis 2013;208(1): p. 10-6.
- Warrener I, Slibinskas R, Chua KB et al. A point of care test for measles diagnosis: detection of measles specific IgM antibodies and viral nucleic acid. Bull Worls health Organ 2011;89:675-82
- WHO. Manual for the Laboratory-based Surveillance of Measles, Rubella, and Congenital Rubella Syndrome. 2018, third edition; Available from: https:// www.who.int/immunization/monitoring_surveillance/burden/laboratory/ manual/en/.
- WHO. Immunization, Vaccines and Biologicals: Measles. Last update: 27 October 2020; Available from: https://www.who.int/immunization/monitoring_ surveillance/burden/vpd/surveillance_type/active/measles/en/.