Parvovirus B19 index
Index of pathogens
[Please click on the initial letter of the pathogen or simply scroll down the list!]
Vossen A
The microorganism and its clinical presentation
Parvovirus B19 is a small non-enveloped single-stranded DNA-virus, which belongs to the family of Parvoviridae. Within the genus Erytroparvovirus, there are 3 genotypes from which Parvovirus B19, also called Erythrovirus, is the predominant human virus [Qiu 2017].
Parvovirus B19 infection may be subclinical (>25%). The most common clinical presentation in children is called erythema infectiosum or “fifth disease”. After an incubation time of 5-20 days (average 7 days) and a period of prodromal symptoms, including fever, coryza, headache, and nausea, a facial erythema (‘slapped cheeks’) appears around 18 days after infection. The rash may then spread to the trunk and limbs. Parvovirus B19 infection may be accompanied by arthralgia or arthritis in less than 10 % in children, but in up to 60% in adults, especially females.
Rare presentations of a parvovirus B19 infection are papular purpuric gloves and socks syndrome, acute hepatitis and virus-associated hemophagocytic syndrome [LCI 2014].
Complications
Parvovirus B19 infection in patients with a short half-life of their red blood cells or decreased erythropoiesis, e.g. spherocytosis or autoimmune hemolytic anemia, or B-thalassemia may lead to a transient aplastic crisis, resulting in severe anemia. Infection in immunocompromised patients may lead to chronic infection and chronic pure red cell aplasia.
Parvovirus B19 infection during the first 20 weeks of pregnancy leads in 5–10% to fetal death or severe fetal anemia, leading to hydrops fetalis.
Epidemiology
The seroprevalence of parvovirus B19 is 2-15% in children between 1 and 5 years of age, 15-60 % between the age of 5 and 9, and between 50-70% in adults [Mossong 2008]. In the Netherlands the seroprevalence in women of childbearing age is around 70% [Gessel 2006]. Around 1% of pregnant women of child-bearing age get infected annually with 5-10% risk of abnormal outcome. During epidemic periods, which occur every 3-5 years, the incidence may increase to up to 10% in susceptible women. Parvovirus is transmitted from person-to-person by contact or via the respiratory route by droplets. Patients are contagious around appearance of the rash but immunosuppressed patients can remain communicable for months and even years. Also, vertical transmission during pregnancy and transmission via blood(products) may occur [LCI 2014].Diagnostic testing
Clinical, viral and serological course is depicted in figure 1.
Figure 1.

Techniques
- Presence of parvovirus DNA in blood by PCR which can be positive during the first months of infection.
- Enzyme immunoassays (=EIA) detecting parvovirus antibodies in blood. EIA’s based on parvovirus B19 capsid proteins (VP1 and VP2) are the currently used antigens for detection of antibodies (sensitivities >98%). For detection of IgM u-capture immunoassays are highly sensitive and specific [Sloots 1996, Enders 2007].
Practical use of serology
Screening
Testing for IgG parvovirus B19 in serum from healthy persons without symptoms of disease is optional for women of childbearing age with increased risk of infection, e.g. working in child care or elementary school. Only test for presence of IgG parvovirus B19.
Infection in immunocompetent child or adult
Indications for testing are exanthema (fifth disease), arthropathy/arthritis, (severe) anemia with low reticulocytes in persons with increased loss or decrease production of red blood cells, For diagnosis of a recent infection: IgM and IgG parvovirus B19 in serum, both a plate or automated EIAs may be used. Consider a “window-fase” (5-7 days) in serology.
Infection in an immunocompromised child or adult
For diagnosis of a chronic infection: PCR parvovirus B19 DNA in plasma or serum should be performed and IgM and IgG parvovirus B19 can be used to measure the specific antibody production.
Infection in a pregnant woman
Indications to test are: signs on ultrasound of fetal hydrops, pregnant women in the first 20 weeks of gestation who have been in contact with a person with fifth disease and optional women of childbearing age with increased risk of infection, e.g. working in child care or elementary school, for screening purposes (only test for presence of IgG). At the time of clinical signs: IgM and IgG parvovirus B19 in serum is recommended. At the time of fetal pathology: IgM and IgG parvovirus B19 should be done, if IgM or IgG positive PCR parvovirus B19 DNA in plasma or serum can support maternal diagnosis. Fetal infection can be diagnosed by parvovirus B19 PCR on amniotic fluid [Gessel 2006].
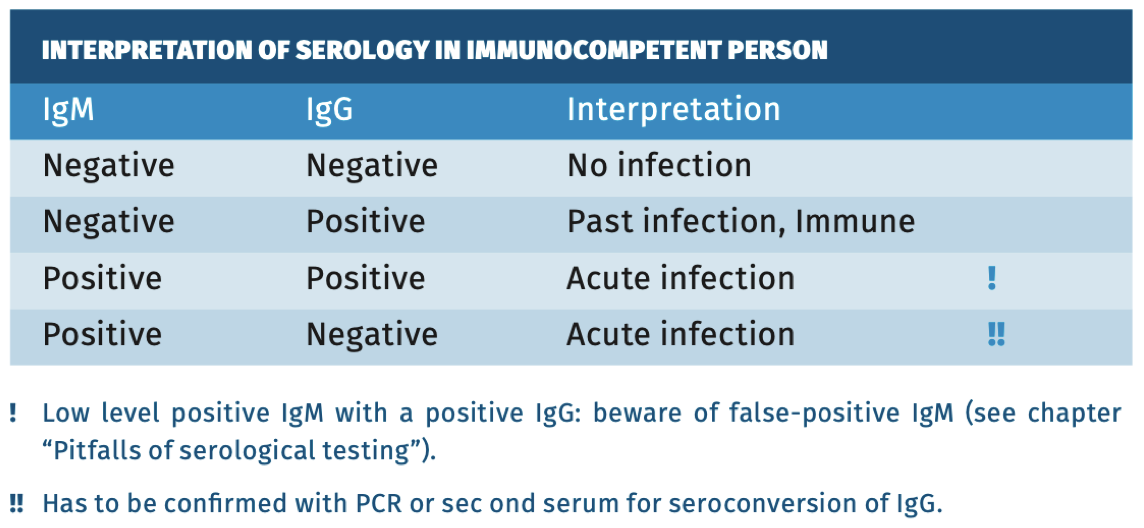
Interpretation of serology
For serological Interpretation in immunocompetent persons see table 1.Table 1.
Sensitivity and specificity
At the time of clinical symptoms (not the prodromal stage) the IgM parvo virus B19 is generally positive. The specificity of IgM parvovirus B19 assays depends on the assays used, with μ-capture EIAs being highly sensitive and specific (>95%) [Sloots 1996].
pitfalls
• Immunocompromised patients: IgM and IgG production is delayed and low or absent. In these patients PCR for parvovirus B19 DNA on serum/plasma should be used as primary diagnostic testing.
• At the time of fetal hydrops the sensitivity of IgM parvovirus B19 is lower (65-70%) [Enders 2007]. When fetal abnormalities are present, the maternal infection has usually been 6-12 weeks sooner. In other words, after maternal infection it takes about 2-3 weeks before the fetus to become infected and then another 4-8 weeks before signs of fetal infection become apparent. Therefore, in case of a negative IgM and a positive IgG, intrauterine parvovirus B19 infection cannot be excluded. PCR parvovirus B19 on maternal blood or amniotic fluid can support the diagnosis.
• After parvovirus B19 infection low-level parvovirus DNA (log 3-4 IU/ ml) can be detected in serum for months to several years [Juhl 2014].
• In immunocompetent individuals serology can also be false negative (IgM and IgG) in the acute infectious stage [Bredl 2011].
References
- Bredl S, Plentz A, Wenzel JJ et al. False negative serology in patients with acute parvovirus infection. J Clin Virol. 2011; 51(2):115-120.
- Enders M, Helbig S, Hunjet A et al. Compa rative evaluation of two commercial enzyme immunoassays for sero diagnosis of human parvovirus B19 infection. J Virol Methods. 2007;146(1-2):409-13.
- Gessel van PH, Gaytant MA, Vossen AC et al. Incidence of parvovirus B19 infection among an unselected population of pregnant women in the Netherlands: A prospective study. Eur J Obstet Gynecol Reprod Biol. 2006;128(1-2):46-9.
- Heegaard ED, Brown KE. Human Parvovirus B19. Clin Micr Rev. 2002;15(3):485-505
- Juhl D, Görg S, Hennig H. Persistence of Parvovirus B19 (B19V) DNA and humoral immune response in B19V-infected blood donors. Vox Sang. 2014;107(3):22632.
- LCI richtlijn parvovirus B19-infectie. https://lci.rivm.nl/richtlijnen/parvovirusb19-infectie 2014.
- Mossong J, Hens N, Friederichs V et al. Parvovirus B19 infection in five European countries: seroepidemiology, force of infection and maternal risk of infection. Epidemiol Infect. 2008;136(8):1059-68.
- Qiu J, Söderlund-Venermo M, Young NS. Human Parvoviruses. Clin Microbiol Rev. 2017;30(1):43-113.
- Sloots T, Devine PL. Evaluation of four commercial enzyme immunoassays for detection of immunoglobulin M antibodies to human parvo virus B19. Eur J Clin Microbiol Infect Dis. 1996;15(9):758-61.

