Hepatitis C virus index
Index of pathogens
[Please click on the initial letter of the pathogen or simply scroll down the list!]
Petit P, Hoeven van der A, Brandenburg A
The microorganism and its clinical presentation
Hepatitis C virus (HCV) is an enveloped positive-sense RNA virus in the genus Hepacivirus. It belongs to the family of Flaviviridae. There is one single serotype but 8 genotypes differing more than 30% in nucleic acid sequence. Different genotypes cause the same disease, but differ in disease severity and in specific susceptibility to antiviral therapy. Some genotypes are ubiquitous (1a, 1b, 2b, 3a), while others are mainly restricted to specific areas and different methods of transmission (drugs, blood) [Gower 2014, Kuiken 2009, Borgia 2018].
The primary and most important feature of HCV infection is its clinical inapparentness: in most cases, symptoms of chronic liver infection only become apparent after decades [Strasak 2011]. 85-90% of acute HCV infections are asymptomatic [Irving 2008]. A minority develops non– specific flare-ups with abdominal pain, fatigue or flu-like symptoms. In less than 10%, signs of hepatitis such as transaminitis or icterus, lasting 3-12 weeks occur [Sagnelli 2014]. Without treatment, 10-40% clear the virus within 6-12 months [Micallef 2006, Scott 2007, Grebely 2014]. The majority of infected persons progress to chronic infection and will have detectable HCV-RNA and HCV-antibodies for years. Recent studies however, show that the prevalence of spontaneous clearance doubled over the past decade and reached 68.7% in 2017 [Seo 2020]. The immune response against HCV is not protective; both reinfections and superinfections occur frequently in high risk groups [LCI 2019]. Persons co-infected with HIV often have a mild infection but only 5-20% will clear the infection without treatment. The remaining 80-95% remain chronically infected getting early complications [LCI 2019]. In recent years, treatment of HCV with direct-acting antivirals (DAA) has become very effective; it has led to sustained virological response in more than 90% of treated persons [Feeney 2014, Pawlotsky 2014].
In chronic infections, extrahepatic symptoms are frequent and diverse; probably related to the persistent presence of HCV-RNA in blood. In 4066% of these chronic infections, the manifestations are related to immune- response (cryoglobulinic vasculitis in 60-90%of patients,
B-cell NHL, Arthralgia, Sicca syndrome) or autoantibodies (RF/ANF in 70% of patients) or inflammation (diabetes mellitus, glomerulone- phritis in 10-20% of patients, or ischaemic heart and cerebrovascular disease) [Cacoub 2016, Younossi 2016].
Complications
- Fulminant infection does not occur!
- Up to 10-20% of chronically infected persons will develop liver cirrhosis after >20 years and 1-5% will develop hepatocellular carcinoma (HCC). Liver failure due to HCV infection is an important cause of liver transplantation worldwide. After transplantation 10- 40% of patients (including patients with sustained viral response) will develop liver cirrhosis anew after 10-15 years due to HCV recurrence [Vinaixa 2013]. This may be less from the start of DAA treatments. In the Netherlands, annually 300-350 persons out of an average of 22,885 chronically infected individuals die from chronic HCV due to cirrhosis or HCC [Hofman 2016].
Epidemiology
HCV infections are ubiquitous; 3% of the world population is infected (=170 million). Humans are the only known reservoir for HCV. Worldwide, almost 400,000 persons die yearly from an HCV infection ( 112,500 in Europe). High prevalence regions (>3.5% of population) include Central Asia, SE Asia, N.Africa, low prevalence regions (<1.5%+) include Japan, N.America and S.America. Seroprevalence in Europe [ECDC 2018]: Germany 0.3-1%, France 0.8-0.9%, Italy 0.6-27.6% , Spain 0.4-1.5% and UK 0.4-1.2%. Seroprevalence in the Netherlands is 0.16%, 75% of infected persons are chronically infected [Koopsen 2018].
In the Netherlands, yearly 30-70 new HCV infections are diagnosed. 2/3 of the chronic infections are found in migrants coming from endemic regions [Urbanus 2011]. Acute infections occur mainly in MSM (men having sex with men) irrespective of their HIV satus. Transmission in IV drug users has been very low since “the syringe change” project [LCI 2019]. Dominant in Europe are HCV genotype 1 (61%) and genotype 3 (30%).
Hepatitis C is a blood-borne infection transmitted by blood-blood contact due to sharing of contaminated needles (in drug use, 60%), high-risk sexual contact (especially in MSM), mother-to-child transmission, contamination during circumcision/scarification/ tattooing with contaminated instruments, or by iatrogenic transmission of blood (products), transplantation of organs/tissue, and rarely through haemodialysis [LCI 2019, Ghany 2009]. In the Netherlands, the transmission risk by needle stick accidents is on average 1.9% [LCI 2019]. Globally 50.000 children [Yeung 2009] each year are born with HCV, infected during pregnancy or perinatally. The vertical transmission rate is approximately 5%. 1/3 of perinatally infected children clear the infection spontaneously, 2/3 need treatment. Success rates are variable depending of the genotype causing the infection (79% type 2-3, 18% type 1). Trials for children with new FDA approved DAA-based treatments are still ongoing [Indolfi 2019]. Maternally-derived antibodies are cleared only after 9-15 months. Therefore, babies born to mothers with an HCV infection should be tested for HCV-RNA in the 2th month of life, and an anti-HCV test at 12-15 months. If the anti-HCV test is positive after 12-15 months, it should be confirmed with HCV-RNA and ALT again.
Diagnostic testing
The clinical, viral and serological course are depicted in figure 1.
Figure 1.
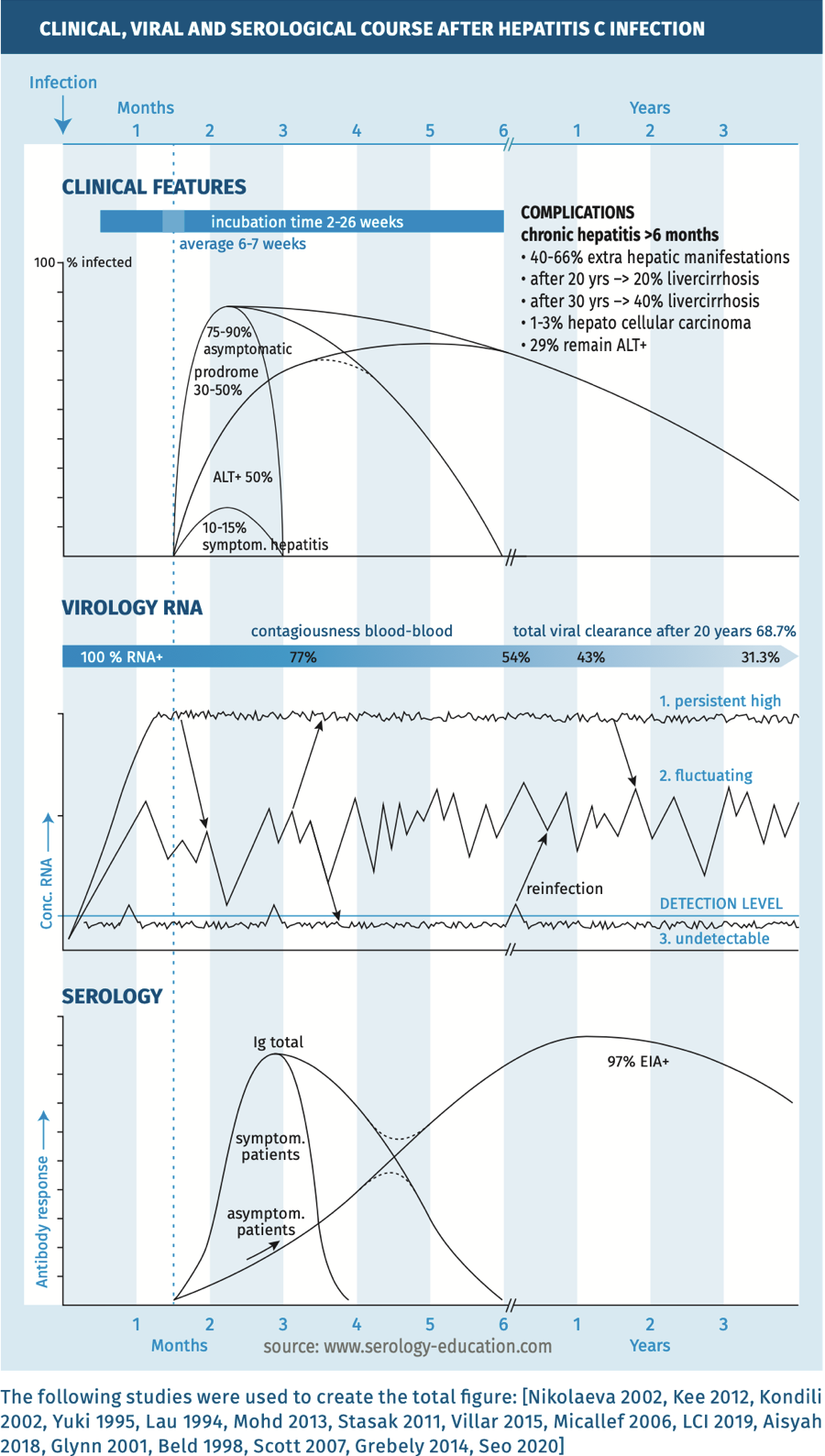 Techniques
Techniques
- Anti HCV serology is highly automated and standardised using a multi-antigen format with immune dominant epitopes from HCV core antigen and non-structural proteins. Most currently used immuno- assays detect all immunoglobulins against HCV independent of isotype, also called total Ig or anti-HCV antibodies in general [Scheiblauer 2006, Alborino 2011, Colin 2001, Cadieux 2016, Villar 2015].
- 4th generation tests that combine the detection of anti-HCV antibodies with detection of hepatitis core antigen have an average time to positivity of 2-4 weeks in acute infection [Irshad 2013, Kamili 2012, Colin 2001, Cadieux 2016], but are not used in the Netherlands.
- RDT/POCT are available [Mane 2019, Tang 2017], but not used in the Netherlands. These are rapid tests that can be performed on saliva and on finger prick bloodsamples. Sensitivity and specificity of these rapid tests are lower than the automated EIA systems. A positive rapid test always needs confirmation with immunoassays or HCVPCR[Shivkumar 2012, Mane 2019]* **.
- The sole detection of IgM or IgA antibodies has no practical value [Gonzalez Quintela 2003].
- Immunoblot: Recombinant Immuno Blot Assay (RIBA) can be used as confirmation of low positive results or to confirm positive anti-HCV tests in low prevalence regions [Villar 2015].
- HCV-RNA /NAT to prove a current infection HCV-PCR test is obligatory.
* HCV core-antigen tests are easy, cheap and fast, and correlate well with HCV-RNA levels but have a lower sensitivity than PCR and therefore are used only for fast testing outside laboratories [Kuo 2012].
** Anti-HCV avidity tests [Sagnelli 2012, Coppola 2007, Villar 2015] distinguishing antibodies of low and high avidity to discriminate between recent (low) and chronic (high) infections are commercially available but are not used in routine diagnostics.
Practical use of serology
Screening
Persons within risk-groups: anti-HCV antibody test. A positive test needs to be confirmed with HCV-RNA or, when HCV-RNA is negative, confirmation with immunoblot to rule out a non-specific reaction is necessary.
Screening in pregnancy and HIV/HBV positives should also include the test with HCV-RNA [Jhaveri 2018, LCI 2019].
Suspected infection in immunocompetent (chronic) persons
See table 1. Antibody levels decrease and are low in patients with chronic infection.
Suspected infection in immunocompromised persons
Immunocompromised persons have a delayed or even an absent antibody response; therefore, an HCV-RNA test should be included to increase sensitivity.
Figure 2.
Interpretation of serology
The interpretation of anti-HCV results in an immunocompetent person is depicted in table 1.
Table 1.
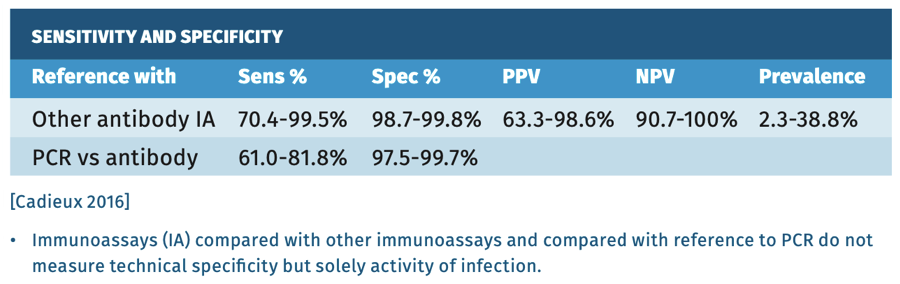
Sensitivity and specificity
There is a high agreement between commercially available immuno- assays and all have equivalent sensitivity and specificity of >99% being positive 4-6 weeks after infection.
Most infected individuals develop detectable antibodies within 3 months, but in immunocompromised persons, immune response can be delayed up to 12 months, or may even be absent. The sensitivity, though, declines rapidly in the first 6 months after infection.
Screening relies on antibody testing, but in persons who have cleared the infection antibodies persist low-level, therefore additional diagnostics, like HCV-RNA, are needed in order to confirm or rule out active disease. Testing in low prevalence regions like Western Europe using tests with 98% specificity, will yield more false-positive results than true-positive results [Cadieux 2016] (see table 2).
Table 2.
pitfalls
- Patients with symptoms of hepatitis should always be checked for other causes of hepatotropic diseases, even when serology shows HCV antibodies, because chronic HCV infection most often progresses asymptomatic [Sagnelli 2014]!
- Positive predictive value in low prevalence populations is low: see general chapter on pitfalls [Ponde 2017, Schleiblauer 2006, Vo 2016].
- False-positive tests in low positive sera: see general chapter on pitfalls.
- Due to high occurrences of re- and super-infections of HCV, screening in patients with known previous infection should be performed with HCV-RNA.
- Seroconversion in HCV can be very late or absent especially in immunocompromised patients, follow up or HCV-RNA test will confirm results. Even in the follow-up results may remain negative!
References
- Aisyah DN, Shalleross L, Hully AL. Assessing hepatitis C spontaneous clearance and understanding associated factors. A systematic review and metaanalysis. J Viral Hepat 2018;25(6):680-98.
- Alborino F, Burighel A, Tiller FW et al. Multicenter evaluation of a fully automated third generation anti-HCV antibody screening test with excellent sensitivity and specificity. Med Microbiol Immunol 2011;200(2):77-83.
- Beld M, Penning M, McMorrow M et al. Different hepatitis C virus (HCV) RNA load profiles following seroconversion among injecting drug users without correlation with HCV genotype and serum alanine aminotransferase levels. J Clin Microbiology 1998;36(4):872-877.
- Boon D, Bruce V, Pathel EU et al. Antibody avidity-based approach to estimate population-level incidence of hepatitis. J Hepatol 2020;73(2):294-302.
- Borgia SM, Hedskog C, Panhy B et al. Identification of a novel hepatitis C virus genotype from Punjab, India: Expanding classification of hepatitis C virus into 8 genotypes. J of Inf Dis 2018;218(11):1722-1729.
- Cacoub P, Comarmond C, Domont F et al. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther Adv Infect Dis 2016;3(1):3-14.
- Cadieux G, Campbell J, Dendukuri N. Systematic review of the accuracy of antibody tests used to screen asymptomatic adults for hepatitis C infection. CMAJ open 2016;4(4):E738-E745.
- CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR may 7, 2013.
- Colin C, Lanoir D, Touzet S et al. Sensitivity and specificity of third generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat 2001;8:87-95.
- Coppola N, Pisapia R, Marrocco C et al. Anti-HCV IgG avidity index in acute hepatitis C. J Clin Virol 2007;40(2):110-115.
- ECDC systematic review. Hepatitis B and C epidemic in selected population groups in EU/EEA. Technical report 2018.
- Feeney ER, Chung RT. Antiviral treatment of hepatitis C. BMJ (Clinical research ed) 2014; 348:g3308.
- Gower E, Estes C, Blach S et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61(1suppl):S45-57.
- Ghany MG, Strader DB, Thomas DL et al. Diagnosis, management and treatment of hepatitis C: an update. Hepatology 2009;49:1335-1374.
- Glynn SA, Wright DJ, Kleinman S et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion 2005;45:994-1002.
- Gonzalez-Quintela A, Alende MR, Gamalla R et al. Serum immunoglobulins (IgG,A,M) in chronic hepatitis C: comparison with non-cirrhotic alcohol liver disease. Hep Gastroent 2003;50(54):2121-6.
- Grebely J, Page K, Sacks-Davis R et al. The effects of female sex, viral genotype and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014;59(1):109-20.
- Hofman R, Nusselder WJ, Veldhuijzen IK et al. Mortality due to viral hepatitis B and C infections in the Netherlands. Ned Tijdschr Geneesk 2016;160:D511.
- Indolfi G, Easterbrook P, Disheiko GM et al. Hepatitis C virus infection in children and adolescents. Lancet Gastroenterol Hepat 2019;4:477-487.
- Irshad M, Mankotia DS, Irshad K. Al insight into the diagnosis and pathogenesis of hepatitis C virus infection. World J Gastroenterol 2013;19(44):7896-7909.
- Irving W, Salmon D, Boucher C et al. Acute hepatitis C virus infection. Euro Surveill 2008;13(21):1-4.
- Haveri R, Broder T, Bhattacharya D et al. Universal screening of pregnant women for hepatitis C: the time is now. Clinical Infectious Diseases 2018;67:14931497.
- Kamili S, Drobeniuc J, Araujo AC et al. Laboratory diagnostics for hepatitis C virus infection. Clin Infect Dis 2012;55(suppl 1):S43-S48.
- Kee KM, Wang JH, Hung CH et al. Decreased anti-HCV titer and associated factors in chronic hepatitis C patients after sustained virological response: a prospective study. J Gastroenterol Hepatol 2012;27:1106-1111.
- Kondili A, Chionne P, Costantino A et al. Infection rate and spontaneous seroreversion of anti-hepatitis C virus during the natural course of hepatitis C virus infection in the general population. Gut 2002;50:693-696.
- Koopsen J, vanSteenbergen JE, Richardus JH et al. Chronic hepatitis B and C infections in the Netherlands: estimated prevalence in risk groups and the general population. Epidemiology and Infection 2018;147(e147):1-8.
- Kuiken C, Simmonds P. Nomenclature and numbering of the hepatitis C virus. Methods Mol Biol 2009;510:33-53.
- Kuo YH, Chang KC, Wang JH et al. Is hepatitis C virus core antigen an adequate marker for community screening? Journal of Clinical Microbiology 2012;50(6):1989-1993.
- Lau GK, Lesniewski R, Johnson RG et al. Immunoglobulin M and A antibodies to hepatitis C core antigen in chronic hepatitis C virus infection. J Med Virol 1994;44:1-4.
- LCI. Hepatitis C. Richtlijn 2019.
- Mane A, Sacks J, Sharma S et al. Evaluation of five rapid diagnostic tests for detection of antibodies to hepatitis C virus(HCV): a step towards scale-up of HCV screening efforts in India. PLoS One 2019;14(1):e0210556.
- Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis infection: a systematic review of longitudinal studies. J Viral Hepat 2006;13(1):34-41.
- Mohd Hanafiah K, Groeger J, Flaxman AD et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013;57: 1333-1342.
- Nikolaeva LI, Blokhina NP, Tsurikova NN et al. Virus specific antibody titres in different phases of hepatitis C virus infection. J Viral Hepat 2002;9:429-437.
- Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies and challenges. Gastroenterology. 2014;146(5):1176–92.
- Ponde R. The serological markers of acute infection with hepatitis A, B, C, D, E and G viruses revisited. Arch Virol 2017;162:3587-3602.
- Sagnelli E, Tonziello G, Pisaturo M et al. Clinical applications of antibody avidity and immunoglobulin M testing in acute HCV infection. Antivir Ther 2012;17(7ptB):1453-1458.
- Sagnelli E, Sagnelli C, Pisaturo M et al. Hepatic flares in chronic hepatitis C:spontaneous exacerbation vs hepatotropic viruses superinfection. World J Gastroenterol 2014; 20(22):6707-6715.
- Scott JD, Gretch DR. Molecular diagnostics of Hepatitis C virus infection: a systematic review. JAMA 2007;297(7): 724-32.
- Scheiblauer H, El-Nageh M, Nick S et al. Evaluation of the performance of 44 assays used in countries with limited resources for the detection of antibodies to hepatitis C virus. Transfusion 2006;46(5):708-718.
- Seo S, Silverberg MJ, Marcus JL et al. Prevalence of spontaneous clearance of hepatitis C virus infection doubled from 1998 to 2017. Clin Gastroenterol Hepatol 2020;18(2): 511-513.
- Shivkumar S, Peeling R, Jafari Y et al. Accuracy of rapid and point-of-care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med 2012;157:558-66.
- Strasak AM, Kim AY, Lauer GM et al. Antibody dynamics and spontaneous viral clearance in patients with acute hepatitis C infection in Rio de Janeiro, Brazil. BMC Infectious Diseases 2011;11(15):1-5.
- Tang W, Chen W, Amini A et al. Diagnostic accuracy of tests to detect Hepatitis C antibody: a meta-analysis and review of the literature. BMC infect Dis 2017;17(Suppl 1):39-57.
- Urbanus AT, Laar van der TJ, Hoek van den A et al. Hepatitis C in the general population of various ethnic origins living in the Netherlands: should nonWestern migrants be screened? J Hepatol 2011;55(6):1207-14.
- Villar LM, Cruz HM, Barbosa JR et al. Update on hepatitis B and C virus diagnosis. World J Virol 2015;4(4);323-342.
- Viniaxa C, Rubin A, Aguilera V et al. Recurrence of hepatitis C after liver transplantation. Annals of Gastroenterology 2013;26:304-313
- Vo MT, Bruhn R, Kaidarova Z et al. A retrospective analysis of false-positive infectious screening results in blood donors. Transfusion 2016;56(2):457465.
- Yeung LTF, Roberts EA. Current issues in the management of paediatric viral hepatitis. Liver International 2009; ISSN 1478-3223.
- Younossi Z, Park H, Henry L et al. Extrahepatic manifestations of hepatitis C: a meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology 2016;150(7):1599-1608.
- Yuki N, Hayashi N, Ohkawa K et al. The significance of immunoglobulin M antibody response to hepatitis C virus core protein in patients with chronic hepatitis. Hepatology 1995;22:402-406.
Keyword(s): Hepatitis C virus



