Coxiella burnetii (Q-fever) index
Index of pathogens
[Please click on the initial letter of the pathogen or simply scroll down the list!]
Hanssen D, Schneeberger P, Petit P
The microorganism and its clinical presentation
Q-fever is a worldwide zoonosis caused by an infection with Coxiella burnetii (C. burnetii). C. burnetii is a Gram-negative intracellular bacterial pathogen related to Legionella pneumophila. It multiplies in phagolysosomes (monocytes and macrophages) of infected cells. The bacterium shows antigenic variation related to mutational variation in its lipopolysaccharide. This phase variation results in the growth of two different morphological types: the large-cell variant (LCV), phase I, and the small-cell variant (SCV), phase ll. The large-cell variant (phase l) is the metabolically active and infectious form found in chronic infections. The small-cell variant (phase II) is less virulent, occurs in the acute infection, and is released during the lysis of infected cells resulting in spore-like forms in the environment. This antigenic shift is used for the serological difference between acute and chronic Q-fever [Fournier 1998]. Humans are primarily infected by inhaling contaminated aerosols [Marrie 1990]. Q-fever has an average incubation period of two weeks (range 2 to 29 days). Most primary C. burnetii infections remain asympto-matic (60%). Symptomatic acute infections present as a non-specific influenza-like illness (25%), pneumonia (25%), hepatitis (45%) [Melenotte 2018] with fever (88-100%) and severe headache (68-98%) in adults [Anderson 2013] and gastrointestinal symptoms (50-80%) with a skin rash (50%) in children. In the Netherlands, 1.3% of community-acquired pneumonia is caused by C. burnetii [Braun NTVG 2004].
Complications
Several months to years after the primary infection, irrespective of whether this primary infection was symptomatic or asymptomatic, a chronic form of Q-fever develops in about 1% of patients due to the persistence of the bacteria [Schneeberger 2014]. Complications of chronic Q-fever can be severe and mainly comprise of endocarditis and vascular infections. Endocarditis occurs in about 35%-75% of persistent infections. Vascular infection occurs in around 19%-57% of chronic Q-fever cases [Tissot-Dupont 2007, Million 2010, Kampschreur 2014]. The prognosis of vascular infections is much worse than endocarditis [Million 2015]. Other less frequent Q-fever complications include osteoarticular infection, cholecystitis, thrombosis, pregnancy-related complications, lymphadenitis, meningoencephalitis, pericarditis, myocarditis, eye involvement, interstitial lung disease, giant cell arteritis, and hemophagocytic syndrome. Q-fever has recently also been associated with the development of lymphoma [Melenotte 2018, Million 2014]. A post-Q-fever fatigue syndrome (PQffs), which is characterised by persistence (> one year) of fatigue, pulmonary disorders, and impairment of general and social functioning, develops in approximately 20% of patients, however without evidence of persistence of the infection by C. burnetii [Morroy 2016]. Complications are severe and underestimated in immunocompromised patients and patients with valvular abnormalities or valvular/vascular prostheses [Melenotte 2016].
EPIDEMIOLOGY
C. burnetii is distributed worldwide and is transmitted to domesticated animals such as sheep, goats, cattle, cats, and dogs [Aitken 1987, Webster 1995]. Sporadic cases, as well as outbreaks, may occur. Humans can be infected through contaminated aerosols released in high concentrations during the parturition of infected goats and sheep [Benenson 1956]. Amniotic fluid and placental tissue contain high numbers of bacteria [Abinanti 1953]. Infection is an occupational hazard if working in proximity to animals or animal products (farmers, veterinarians, abattoir workers, animal handlers). Studies carried out on the role of consumption of infected raw dairy products are inconclusive [Aitken 1987, Benson 1963, Krumbiegel 1970]. However, consuming raw milk and raw milk products contaminated with
C. burnetii has been associated with seroprevalence in humans, and some studies report infection after consuming contaminated raw milk [Maltezou 2004, Signs 2011]. Therefore the risk of C. burnetii infection by consuming unpasteurized milk and dairy products may not be negligible. Case reports of person-to-person transmission and vertical trans-mission have been described, as well as transmission through sexual intercourse, blood transfusion, and organ or bone marrow trans-plantations [Deutsch 1950, Raoult 1994, Maurin 1999, Kanfer 1988].
C. burnetii can survive for long periods in the environment and is resistant to disinfectants and dehydration [EFSA 2010]. Airborne transmission is the most powerful predictor for observed human Q-fever incidence rates. Vegetation, soil moisture, and arable land are risk factors for the transmission of C. burnetii [Leuken 2016]. The infectious dose for 50% human illness (ID50) is about 1.18 bacteria exposure [Brooke 2013]. Infections with C. burnetii are most often described in the 45-64 years age group, followed by those aged 25-44 and those aged over six years old. Men are more often infected than women. An increased incidence is observed during summer due to the spring lambing season [EFSA 2010].
Patients with cardiac valve defects, aneurysms, and vascular anomalies or prostheses are more at risk of developing chronic Q-fever [Kampschreur 2012]. These patients need to be checked for chronic Q-fever with serology, PCR, transthoracic echocardiography (TTE) or PET scan whenever clinical suspicion or inexplicable symptoms occur. Men aged over 40 years with valvulopathies are especially at risk of progression to chronic endocarditis [Million 2013].
Diagnostic testing
Clinical, bacterial and serological course is depicted in figure 1.
Figure 1.
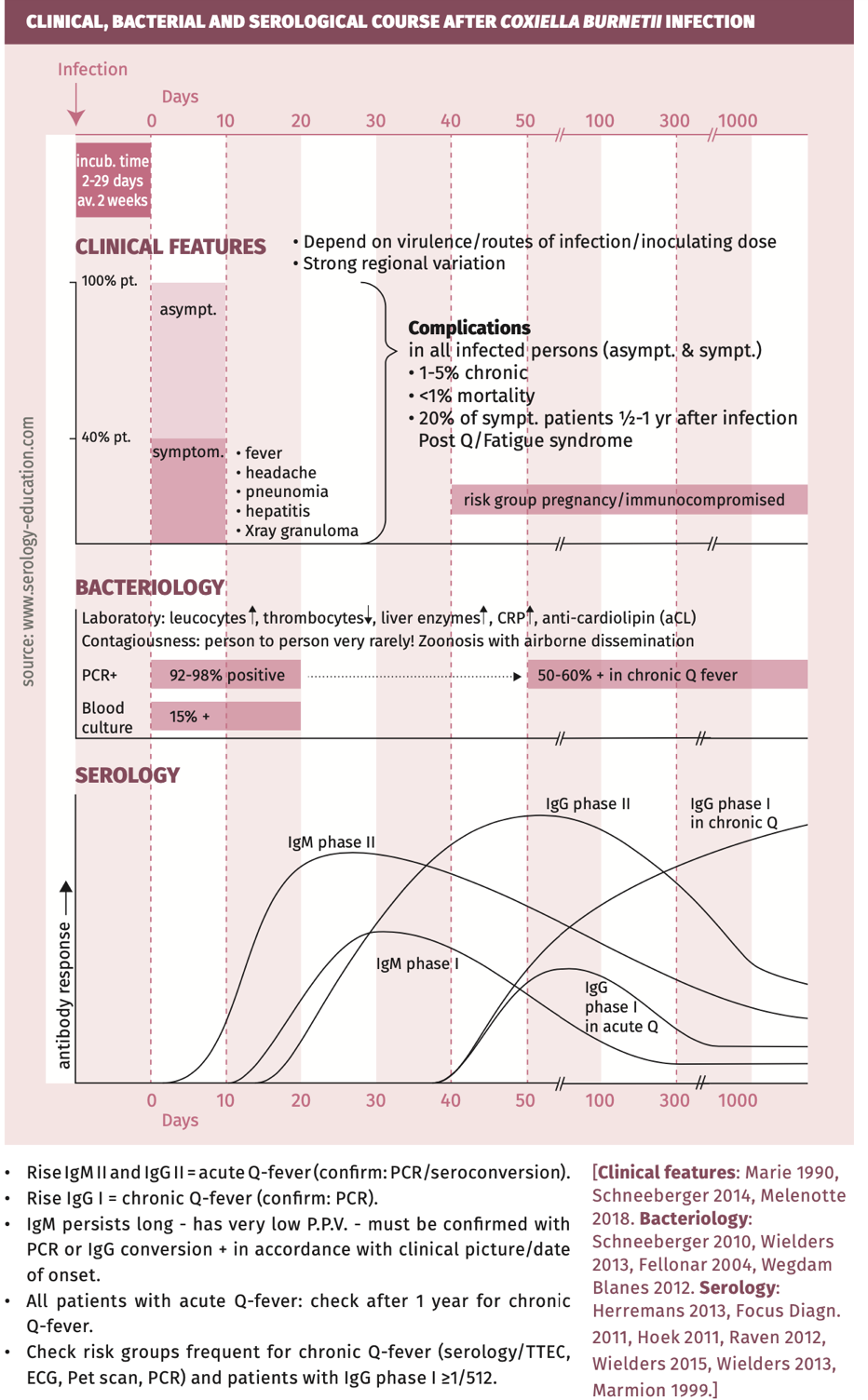
Techniques
- CULTURE: only positive in 15% of acute Q-fever patients. Special media and conditions are necessary, with laborious techniques that require working in biosafety level 3 conditions (highly infectious).
- PCR: C. burnetii DNA can be detected in blood up to 17 days after the onset of clinical signs and symptoms. PCR has high sensitivity and specificity for diagnosing acute Q-fever [Schneeberger 2010, Wielders 2013] in serum and tissue, but sensitivity is very low on sputum/throat samples. In chronic Q-fever, PCR is less sensitive [Wegdam-Blans 2012, Fenollar 2004] (see table 2).
- SEROLOGY: Because most samples will not be collected within two weeks after the first day of illness, in which C. burnetii DNA is still detectable, the diagnosis of recent Q-fever is mainly based on serology.
- IFA (indirect immunofluorescence assay), ELISA (enzyme-linked immunosorbent assay), and CFA (complement fixation assay) are all suitable serodiagnostic assays for diagnosing acute Q-fever with comparable sensitivities and specificities [Herremans 2013]. CFA combines IgM and IgG antibodies. ELISA, IFA and CFA can detect IgM and IgG separately, and recognise the different phases of Q-fever (phases I and II). IFA is considered the reference method of laboratory diagnosis of Q-fever but is very labour intensive and subjective but essential in epidemiological studies and differentiation of the two phases of Q-fever (see table 2). ELISA can be an alternative for screening large sample numbers.
- Analysis of IgM phase I/II and IgG I/II makes it possible to identify the various stages of Q-fever infection (see figure 1 and table 1).
- IGRA: There is always a small number of patients (1.1% four years after infection [Wielders 2015]) who will test negative some time after the infection. IGRA (whole blood “interferon y release”) is then a more sensitive marker than antibody response [Scholzen 2021].
Practical use of serology
There are 2 groups of patients: patients who develop an acute Q-fever infection, and patients who are at risk for a complicated Q-fever infection (see also algorithm figure 2).
Figure 2.

Screening
To test for Coxiella burnetii-specific antibodies to establish infection dependent or independent of symptoms, an IgG II test is sufficient, and will be positive in the acute (high titre) and in the chronic (low titre) phase (see figure 1) [Dupont 1994] for years up to lifelong. There is much discussion in low endemic areas about screening in risk groups. Risk groups are people with aortic aneurysms, endovascular protheses and heart-valve surgery [Wielders 2013], and also goat-keepers (Australia) and in some countries pregnant women (France). In high endemic areas or countries, it could be justifiable to screen only patients with FUO or endocarditis of unknown cause [Wielders 2013].
Suspected infection in immunocompetent patients
ln the acute phase of Q-fever (first two weeks) PCR is the most sensitive test.
After two weeks C. burnetii DNA declines. Even then PCR may to be done but serology has to be added for sensitivity and to prove the phase (I or II) and kind of Immunoglobulins (IgM and IgG) for the diagnosis of acute or chronic Q-fever. Serological acute Q-fever is diagnosed by detecting IgM phase II, and/or seroconversion or a significant rise in titre of IgG. A solitary IgM phase II has a low positive predictive value for acute Q-fever due to the long persistence of IgM phase II antibodies [Raven 2012]. This is the case in endemic areas or after an epidemic period, and always needs to be confirmed by additional testing (IgG) and if possible PCR. A titre rise in IgG II is relevant in acute phase and stays positive for years up to lifelong. IgG I appears later in infection!
ELISA and IFA measuring IgG phase II can detect seroconversion 10 to 15 days post-infection [Maurin 1999]. However, due to the rapid time to peak, a significant rise in titre is rarely measured in paired serum samples [Wielders 2015].
Screening for aortic aneurysms must be considered in all positive Q-fever patients because of the high risk for the development of complications [Eldin 2017].
Suspected chronic infection in immunocompromised patients
Acute Q-fever patients should be serologically screened at least once during the first year following acute infection [Wielders 2013]. Yearly follow-up is recommended for patients without risk factors having an initial IgG phase I titre above 1:512 and for patients with known risk factors for chronic Q-fever (see complications). Diagnosis of chronicity is associated with the titre of IgG I (phase one response) which is a better marker than IgG II. In patients with proven endocarditis or vascular infection, chronic Q-fever must be excluded or confirmed by combined serology and PCR (PCR+ and/or positive serology IgG phase I ≥1:1024) [Hoek vder 2011]. However, the sensitivity of PCR in chronic Q-fever is suboptimal (50-60%). A probable chronic Q-fever infection is diagnosed in cases with a solitary positive IgG phase I ≥1:1024 in patients with risk factors or symptoms such as vasculopathy, vascular aneurysm, hepatitis, osteomyelitis, signs of chronic infection, lymphadenitis, histological signs of granulomatous disease, immune disorder, or pregnancy (sensitivity nearly 100% and PPV 98% ). Early detection of chronic Q-fever is essential for timely treatment since chronic Q-fever leads to complications in up to 60% of patients and has 15% mortality [Million 2010, Roeden van 2018]. In culture negative endocarditis, TTE and alpha-Cardiolipin IgG (alphaCl) are strong indicators for Q-fever endocarditis in France [Melenotte 2018].
Post-Q-fever fatigue syndrome (PQffs)
There are no bacteriological tests or other tests that can be used to diagnose Q-fever-related chronic fatigue and long-term suspicious symptoms.
Interpretation of serology
Table 1.
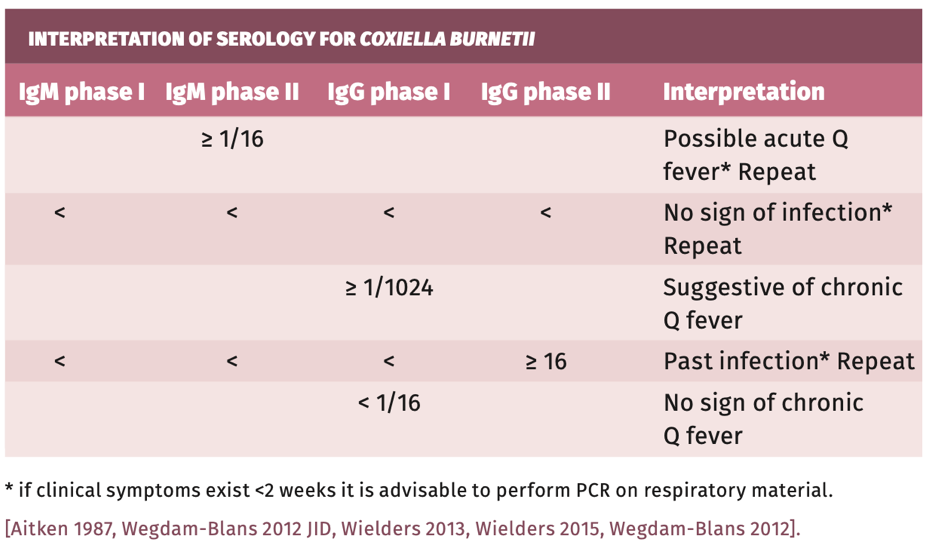
SENSITIVITY AND SPECIFICITY
Follow-up serology:
- If all results are the same asthe first serum: no sign of infection.
- If there is a significant rise in any titre, it is suggestive of recent infection.
Results acute and chronic Q-fever with IFA, ELISA, and CF are presented in table 2.
Table 2.

pitfalls
- Ticks are known to harbor C. burnetii and may act as vectors to transmit the bacterium to wildlife. Ticks also frequently carry Coxiella-like bacteria. These Coxiella-like bacteria are not transmitted to other animals. When ticks are screened for C. burnetii, Coxiella-like bacteria can yield false positive C. burnetii PCR results [WegdamBlans 2012].
- Serologic cross-reactions have been described between C. burnetii and Legionella spp., Bartonella spp, Ehrlichia spp., and Rickettsia rickettsia [Maurin 1999, Graham 2000]. Pregnancy and an acute Epstein-Barr virus infection also can cause false-positive test results [La Scola 1996, Musso 1997].
- Immune activation by Q-fever also causes cross-reactions with other laboratory tests for autoimmune and infectious agents (HIV, Brucella).
- See also general chapter "Pitfalls".
References
- Abinanti FR, Lennette EH, Winn JF et al. Q fever studies. XVIII. Presence of Coxiella burnetii in the birth fluids of naturally infected sheep. Am J Hyg. 1953;58(3):385-8.
- Aitken ID, Bogel K, Cracea E et al. Q fever in Europe: current aspects of aetiology, epidemiology, human infection, diagnosis and therapy. Infection. 1987;15(5):323-7.
- Benenson AS, Tigertt WD. Studies on Q fever in man. Trans Assoc Am Physicians. 1956;69:98-104.
- Benson WW, Brock DW, Mather J. Serologic Analysis of a Penitentiary Group Using Raw Milk from a Q Fever Infected Herd. Public Health Rep. 1963;78:707-10.
- Braun JJ, Graaff de CS, Goey de J et al. Buiten het ziekenhuis opgelopen pneumonie: verwekkers en beloop bij patienten opgenomen in een algemeen ziekenhuis. NTvG 2004;148:836-40.
- Brooke RJ, Kretzschmar ME, Mutters NT et al. Human dose response relation for airborne exposure to Coxiella burnetii. BMC Infect Dis. 2013;13:488.
- Broos PP, Hagenaars JC, Kampschreur et al. Vascular complications and surgical interventions after world's largest Q fever outbreak. J Vasc Surg. 2015;62(5):1273-80.
- Deutsch DL, Peterson ET. Q fever: Transmission from one human being to others. J Am Med Assoc. 1950;143(4):348-50.
- EFSA Panel on Animal Health and Welfare (AHAW). Scientific Opinion on Q fever EFSA Journal 2010. 2010;8(5): 1595.
- Eldin C, Melenotte C, Mediannikov O et al. From Q Fever to Coxiella burnetii Infection: a Paradigm Change. Clin Microbiol Rev. 2017;30(1):115-90.
- Fenollar F, Fournier PE, Raoult D. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection. J Clin Microbiol. 2004;42(11):4919-24.
- Focus Diagnostics. Q Fever IFA IgG: Indirect immunofluorescent assay (IFA) for the detection of human IgG antibodies to Coxiella burnetii. Focus Diagnostics, Cypress, CA, USA; 2011.
- Graham JV, Baden L, Tsiodras S et al. Q fever endocarditis associated with extensive serological cross-reactivity. Clin Infect Dis. 2000;30(3):609-10.
- Herremans T, Hogema BM, Nabuurs M et al. Comparison of the performance of IFA, CFA, and ELISA assays for the serodiagnosis of acute Q fever by quality assessment. Diagn Microbiol Infect Dis. 2013;75(1):16-21.
- Hoek vder W, Versteeg B, Meekelenkamp JC et al. Follow-up of 686 patients with acute Q fever and detection of chronic infection. Clin Infect Dis. 2011;52(12):1431-6.
- Kampschreur LM, Dekker S, Hagenaars JC et al. Identification of risk factors for chronic Q fever, the Netherlands. Emerg Infect Dis. 2012;18(4):563-70.
- Kampschreur LM, Delsing CE, Groenwold RH et al. Chronic Q fever in the Netherlands 5 years after the start of the Q fever epidemic: results from the Dutch chronic Q fever database. J Clin Microbiol. 2014;52(5):1637-43.
- Kanfer E, Farrag N, Price C et al. Q fever following bone marrow transplantation. Bone Marrow Transplant. 1988;3(2):165-6.
- Krumbiegel ER, Wisniewski HJ. Q fever in the Milwaukee area. II. Consumption of infected raw milk by human volunteers. Arch Environ Health. 1970;21(1):63-5.
- La Scola B, Raoult D. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol. 1996;34(9):2270-4.
- Leuken v JPG, Swart AN, Brandsma J et al. Human Q fever incidence is associated to spatiotemporal environmental conditions. One Health. 2016;2:77-87.
- Maltezou HC, Constantopoulou I, Kallergi C et al. Q fever in children in Greece. Am J Trop Med Hyg. 2004;70(5):540-4.
- Marmion BP. Q fever. Medimedia Communications St Leonards NSW Australia 1999.
- Marrie TJ. Q fever - a review. Can Vet J. 1990;31(8):555-63.
- Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12(4):518-53.
- Melenotte C, Protopopescu C, Million M et al. Clinical Features and Complications of Coxiella burnetii Infections From the French National Reference Center for Q Fever. JAMA Netw Open. 2018;1(4):e181580.
- Million M, Thuny F, Richet H et al. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis. 2010;10(8):527-35.
- Million M, Walter G, Thuny F et al. Evolution from acute Q fever to endocarditis is associated with underlying valvulopathy and age and can be prevented by prolonged antibiotic treatment. Clin Infect Dis. 2013;57(6):836-44.
- Million M, Raoult D. Recent advances in the study of Q fever epidemiology, diagnosis and management. J Infect. 2015;71 Suppl 1:S2-9.
- Million M, Roblot F, Carles D et al. Reevaluation of the risk of fetal death and malformation after Q Fever. Clin Infect Dis. 2014;59(2):256-60.
- Morroy G, Keijmel SP, Delsing CE et al. Fatigue following Acute Q-Fever: A Systematic Literature Review. PLoS One. 2016;11(5):e0155884.
- Musso D, Raoult D. Serological cross-reactions between Coxiella burnetii and Legionella micdadei. Clin Diagn Lab Immunol. 1997;4(2):208-12.
- Raoult D, Stein A. Q-Fever during Pregnancy - a Risk for Women, Fetuses, and Obstetricians. New Engl J Med. 1994;330(5):371-.
- Raven CF, Hautvast JL, Herremans T et al. Solitary IgM phase II response has a limited predictive value in the diagnosis of acute Q fever. Epidemiol Infect. 2012;140(11):1950-4.
- Roeden v SE, Wever PC, Kampschreur LM et al. Chronic Q fever-related complications and mortality: data from a nationwide cohort. Clin Microbiol Infect. 2018.
- Scholzen A, Vries de M, Duerr HO et al. Whole blood interferon y release is a more sensitive marker of prior exposure to Coxiella burnetii than are antibody responses. Front Immunol 2021;12:701811
- Schneeberger PM, Hermans MH, van Hannen EJ et al. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin Vaccine Immunol. 2010;17(2):286-90. Schneeberger PM, Wintenberger C, van der Hoek W, Stahl JP. Q fever in the Netherlands - 2007-2010: what we learned from the largest outbreak ever. Med Mal Infect. 2014;44(8):339-53.
- Tissot-Dupont H, Vaillant V, Rey S et al. Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis. 2007;44(2):232-7.
- Todkill D, Fowler T, Hawker JI. Estimating the incubation period of acute Q fever. Epid.Inf.2018;146(6):665-672 Signs KA, Stobierski MG, Gandhi TN. Q fever cluster among raw milk drinkers in Michigan, 2011. Clin Infect Dis. 2012;55(10):1387-9.
- Webster JP, Lloyd G, Macdonald DW. Q fever (Coxiella burnetii) reservoir in wild brown rat (Rattus norvegicus) populations in the UK. Parasitology. 1995;110 ( Pt 1):31-5.
- Wegdam-Blans MC, Kampschreur LM, Delsing CE et al. Chronic Q fever: review of the literature and a proposal of new diagnostic criteria. J Infect. 2012;64(3):247-59.
- Wegdam-Blans MC, Wielders CC, Meekelenkamp J et al. Evaluation of commonly used serological tests for detection of Coxiella burnetii antibodies in well-defined acute and follow- up sera. Clin Vaccine Immunol. 2012;19(7):1110-5.
- Wielders CC, Teunis PF, Hermans MH et al. Kinetics of antibody response to Coxiella burnetii infection (Q fever): Estimation of the seroresponse onset from antibody levels. Epidemics. 2015;13:37-43.
- Wielders CC, Morroy G, Wever PC et al. Strategies for early detection of chronic Q-fever: a systematic review. Eur J Clin Invest. 2013;43(6):616-39.
- Wielders CC, Wijnbergen PC, Renders NH et al. High Coxiella burnetii DNA load in serum during acute Q fever is associated with progression to a serologic profile indicative of chronic Q fever. J Clin Microbiol. 2013;51(10):3192-8.
Keywords: Coxiella burnetii, Q-fever, Queensland fever, Queer fever

