Hepatitis B virus index
Index of pathogens
[Please click on the initial letter of the pathogen or simply scroll down the list!]
Vossen A
The microorganism and its clinical presentation
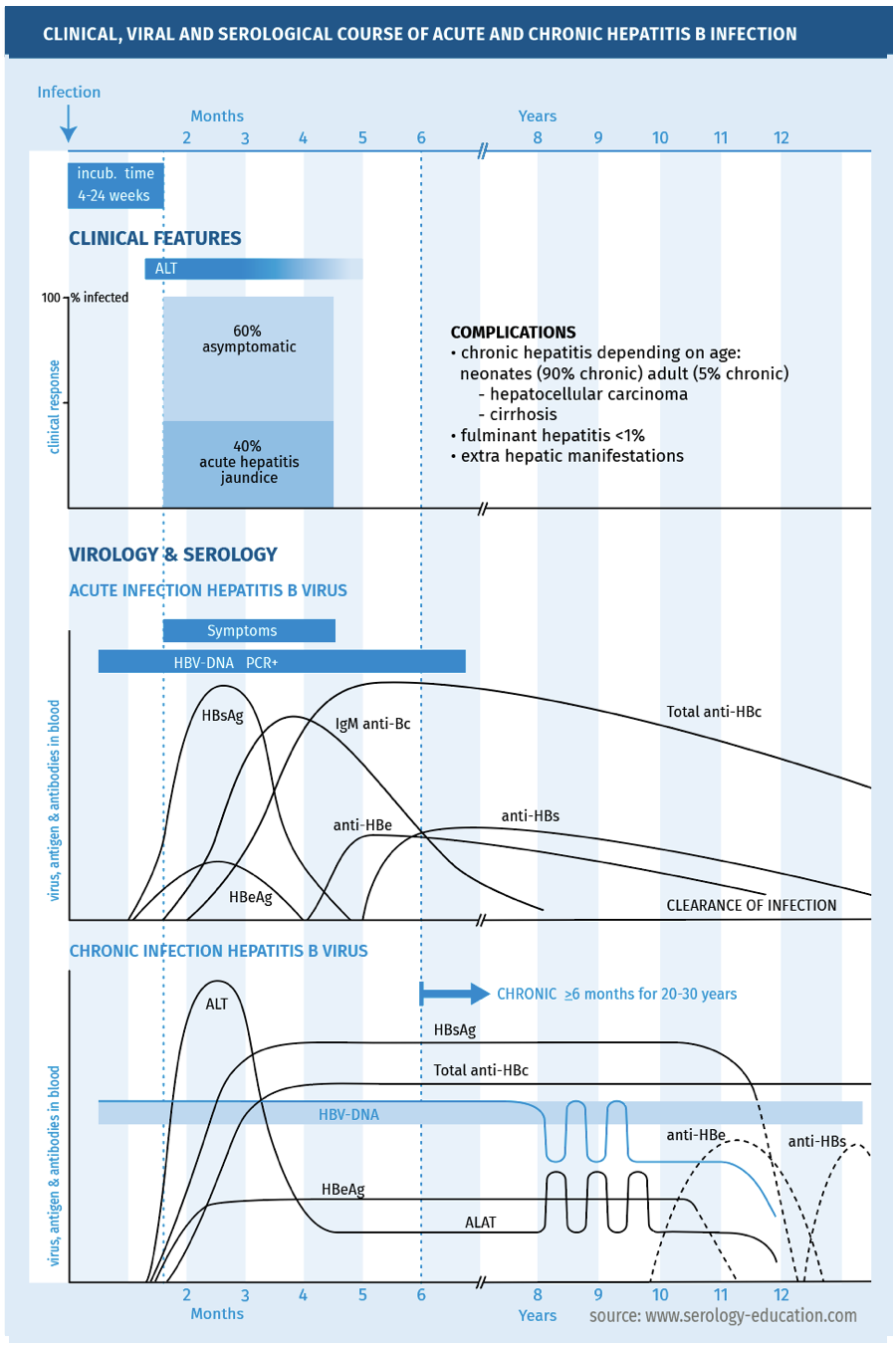
Hepatitis B virus (HBV) is a small enveloped virus, with a circular partially double-stranded DNA genome. It belongs to the family of hepadnaviridae. HBV infection is subclinical in 60 % of immunocompetent adults and in 90% of immunocompetent children. In case of symptoms, an acute infection causes an acute viral hepatitis, after an incubation time of 4 weeks to 6 months. Common symptoms are malaise, loss of appetite, nausea and vomiting, mild fever, dark urine and jaundice. The host immune response is responsible for damage to the virus-infected hepatocytes and for viral clearance. In some patients, HBV infection persists and the acute infection evolves into a persistent, chronic HBV infection, defined as HBsAg-positivity for 6 months or more. The risk of chronic infection is high in perinatally infected children (> 90%), lower in children between 1 and 5 years of age (10-20 %) and low in adults (5%) [McMahon 1985]. There is an increased risk of chronic infection in immunocompromised persons. Several phases of chronic hepatitis B are recognised, depending on HBeAg-positivity, HBV DNA levels, and the course of ALT-levels, namely an immunotolerant phase, immune-active phase and an inactive phase. Chronic hepatitis B may be asymptomatic, but can also be associated with fatigue and symptoms associated with hepatitis. Chronic hepatitis B can develop into liver cirrhosis and hepatocellular carcinoma [Wong 2006, Lok 2007].
Complications
- Acute HBV infection can present in <1% as acute fulminant hepatic failure, with high mortality.
- Chronic hepatitis B can develop into liver cirrhosis and hepatocellular carcinoma.
- Extrahepatic manifestations of hepatitis B are probably due to circulating immune complexes. The acute phase of HBV infection may be accompanied by a serum-sickness-like syndrome with fever, rash, and arthralgia or arthritis, which usually subsides after the onset of jaundice. Two major extrahepatic complications of chronic hepatitis B are polyarteritis nodosa and HBV-associated nephropathy, usually caused by membranous glomerulonephritis [Han 2004].
- In persons with chronic or past HBV infection, HBV can reactivate in case of immunosuppression. HBV reactivation can cause mild symptoms but can also result in fulminant hepatic failure and death. Prior to specific categories of immunosuppressive therapy, HBV screening should be performed and if indicated antiviral prophylaxis or adequate monitoring should be started [Gentile 2017, Loomba 2017].
- In case of co-infection of HBV and HCV, treatment of chronic hepatitis C with direct-acting antiviral agents (DAA) may lead to HBV reactivation. HBV screening is advised before starting DAA therapy [Mahale 2017, Poola 2021].
Epidemiology
HBV is transmitted by exposure to blood or other infectious body fluids. The modes of transmission include blood-blood contact, for example needle stick accidents, sexual transmission and vertical transmission during delivery. HBV infection can be prevented by vaccination.
Hepatitis B is a global problem, with an estimated 350 million HBV carriers. There are areas with >8% chronic infection (high-endemic areas) such as China, South East Asia and Africa, moderate prevalence areas where 2-7% of the people are infected such as Eastern Europe, Russia and Japan, and low-prevalence areas with <2% chronic HBV such as the United States and Western Europe. The estimated prevalence of chronic HBV in the Netherlands is 0.2 %, whereas 2.1 % has a past HBV infection. In 2006 1.5 per 100,000 inhabitants of the Netherlands had acute hepatitis B [LCI richtlijn Hepatitis B].
Diagnostic testing
The clinical, viral and serological course are depicted in figure 1.
FIGURE 1.
Techniques
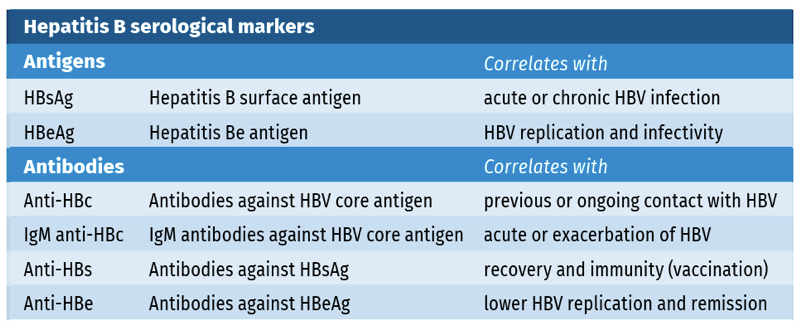
Hepatitis B serology is based on the detection of several HBV antigens and antibodies (see table 1).
Table 1.
- HBV serology is performed using immunoassays, such as Chemiluminescent Immune Assay (CLIA) on automated analysers.
- Most of these assays are qualitative. Anti-HBs is measured in a quantitative assay and expressed as International Units per litre (IU/l). HBsAg can also be measured using a quantitative assay and is expressed as IU/ml.
- A novel biomarker, Hepatitis B core-related antigen (HBcrAg) correlates with serum HBV DNA and intrahepatic covalently closed circular DNA and may predict the development of hepatocellular carcinoma. This assay is still mostly used in research settings (Inoue 2020)
- Besides HBV serology, (quantitative) detection of HBV DNA is used to assess HBV replication. HBV DNA levels, measured in IU/ml, correspond with replication level and contagiousness.
Practical use of serology
Screening
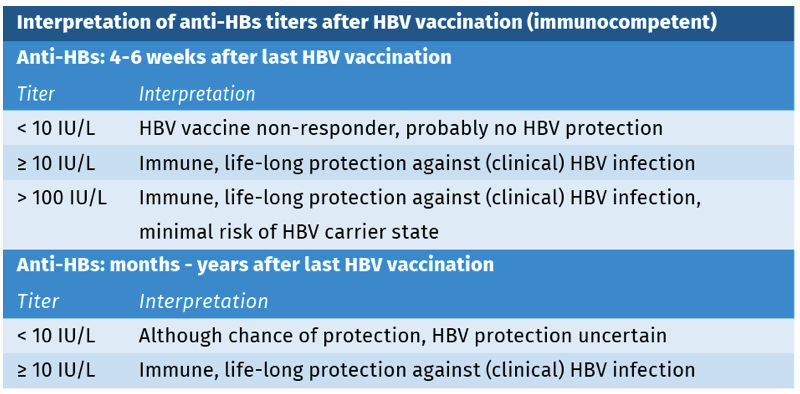
Testing for immunity after HBV vaccination or possible past infection is done by anti-HBs testing (see figure 2). The same algorithm can be used for screening for hepatitis B; although for different goals, different screening assays may be used. In pregnant women and in case of a needle stick injury, screening is performed by testing HBsAg only, whereas in other groups, such as organ or stem cell donors, or persons requiring immunosuppressive therapy, screening of both HBsAg and anti-HBc is advised.
Suspected infection or in immunocompetent child or adult
- In case of clinical suspicion of acute or chronic hepatitis B, serological diagnostics can be performed using a serological algorithm (see figure 2).
Figure 2.

- The same algorithm can be used for screening for hepatitis B; although for different goals, different screening assays may be used. In pregnant women and in case of a needle stick injury, screening is performed by testing HBsAg only, whereas in other groups, such as organ or stem cell donors, or persons requiring immunosuppressive therapy, screening of both HBsAg and anti-HBc is advised.
Suspected infection in immunocompromised child or adult
- In immunocompromised persons, HBsAg can be used for hepatitis B diagnostics, since its production does not depend on an adequate immune-response. Also, in the acute phase, PCR HBV DNA has virtually no added value. Anti-HBc production may be delayed and/or reduced by immunosuppression.
- HBV reactivation is diagnosed by detection of HBsAg seroreversion (HBsAg positivity after being HBsAg negative), or by detection or increase of HBV DNA levels.
Follow-up antiviral treatment of chronic hepatitis B
- Seroconversion of HBeAg to anti-HBe and of HBsAg to anti-HBs are used as the endpoint of therapy [Liu 2018].
- Baseline quantitative HBsAg and decline in HBsAg level can be used to predict HBe-seroconversion and sustained virological response to Peg-IFN-α2a treatment [Liu 2018].
Interpretation of serology
The interpretation of anti-HBV serology markers results for immunocompetent persons is depicted in table 2 and 3 (see also general pitfalls).
Table 2.
Table 3.
Isolated positivity of HBsAg is a rare serological profile which should first be confirmed by neutralising HBsAg. If HBsAg is true-positive, this finding may be explained by very early sampling after infection, antigenemia after hepatitis B vaccination, immunotolerance to HBcAg, or other rare conditions [Pondé 2011]. The level of IgM anti-HBc, measured in a semi-quantitative way, can be used to distinguish acute hepatitis B from an exacerbation of chronic hepatitis B. The optimal cut-off for discrimination depends on the assay used [Pondé 2016].
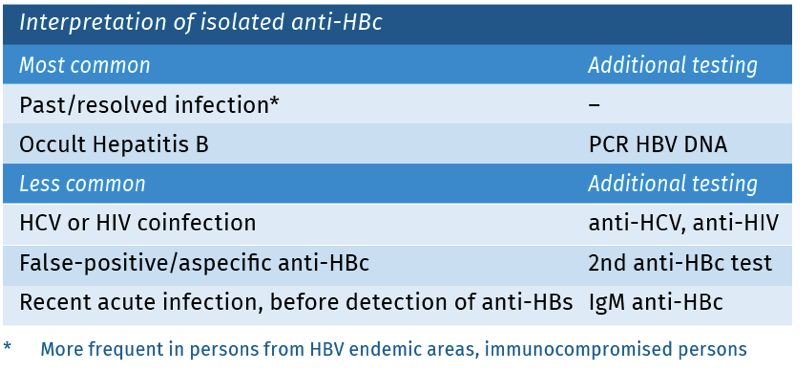
The finding of a positive total anti-HBc, in absence of a positive HBsAg and anti-HBs, is considered ‘an isolated anti-HBc’. There are multiple possible explanations for this pattern [Wang 2017]. See table 4.
Table 4.
Sensitivity and specificity
HBsAg detection has a high analytical sensitivity and performs well in comparison to HBV DNA detection in seroconversion panels, with some weeks delay between HBV DNA and HBsAg [Zaaijer 2001]. The specificity of HBsAg assays is very high (>99%) [van Roosmalen 2006].
pitfalls
- Low HBsAg can be found in blood for a short period after HBV vaccination.
- Low HBsAg can sometimes not be reliably confirmed with a neutralisation assay. Using a second immunoassay or testing for HBV DNA will help to exclude HBV infection.
- After HBV vaccination, anti-HBs titers may decline to undetectable levels. The vaccinated person, however, may still be protected.
- With an adequate antibody response after vaccination (anti-HBs ≥10 IU/l) healthy persons are most likely protected for life against Hepatitis B.
References
- Gentile G, Andreoni M, Antonelli G et al. Screening, monitoring, prevention, prophylaxis and therapy for hepatitis B virus reactivation in patients with haematologic malignancies and patients who underwent haematologic stem cell transplantation: a systematic review. Clin Microbiol Infect. 2017.
- Han SH. Extrahepatic manifestations of chronic hepatitis B. Clin Liver Dis. 2004 May;8(2):403-18.
- Inoue T, Tanaka Y. Novel biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol. 2020 Jul;26(3):261-279. LCI richtlijn Hepatitis B: https://lci.rivm.nl/richtlijnenhepatitisb#epidemiologie
- Liu YY, Liang XS. Progression and status of antiviral monitoring in patients with chronic hepatitis B: From HBsAg to HBV RNA. World J Hepatol. 2018 Sep 27;10(9):603-611.
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007 Feb;45(2):507-39.
- Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017 May;152(6):1297-1309.
- Mahale P, Glenn JS, O’Brien TR. Hepatitis B Virus reactivation associated with direct-acting antiviral therapy for chronic hepatitis C virus. Ann Intern Med. 2017 Nov 21;167(10):759-760.
- McMahon BJ, Alward WL, Hall DB et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985 Apr;151(4):599-603.
- Pondé RA. The underlying mechanisms for the “isolated positivity for the hepatitis B surface antigen (HBsAg)” serological profile. Med Microbiol Immunol. 2011 Feb;200(1):13-22.
- Pondé RA. Acute hepatitis B virus infection or acute exacerbation of chronic hepatitis B infection: the differential serological diagnosis. Eur J Clin Microbiol Infect Dis. 2016 Jan;35(1):29-40.
- Poola S, Sanaka S, Sewell K et al. Hepatitis B surface antibody titres and hepatitis B reactivation with direct-acting antiviral therapy for hepatitis C. J Viral Hepat. 2021 Feb;28(2):373-382.
- van Roosmalen MH, de Jong JJ, Haenen W et al. A new HBsAg screening assay designed for sensitive detection of HBsAg subtypes and variants. Intervirology. 2006;49(3):127-32.
- Wang Q, Klenerman P, Semmo N. Significance of anti-HBc alone serological status in clinical practice. Lancet Gastroenterol Hepatol. 2017 Feb;2(2):123134.
- Wong SN, Lok AS. Treatment of hepatitis B: who, when, and how? Arch Intern Med. 2006 Jan 9;166(1):9-12.
- Zaaijer HL, Vrielink H, Koot M. Early detection of hepatitis B surface antigen and detection of HBsAg mutants: a comparison of five assays. Vox Sang. 2001 Nov;81(4):219-21.
Keyword(s): Hepatitis B virus