Mumps virus index
Index of pathogens
[Please click on the initial letter of the pathogen or simply scroll down the list!]
Bodewes r, Voordouw B, Petit p
The microorganism and its clinical presentation
Mumps is a contagious respiratory viral infection caused by the mumps virus. Mumps virus (MuV) is an enveloped RNA virus that belongs to the genus Orthorubulavirus in the family Paramyxoviridae [Hviid 2008, ICTV 2020]. Humans are the only natural host of MuV [Rubin 2015].
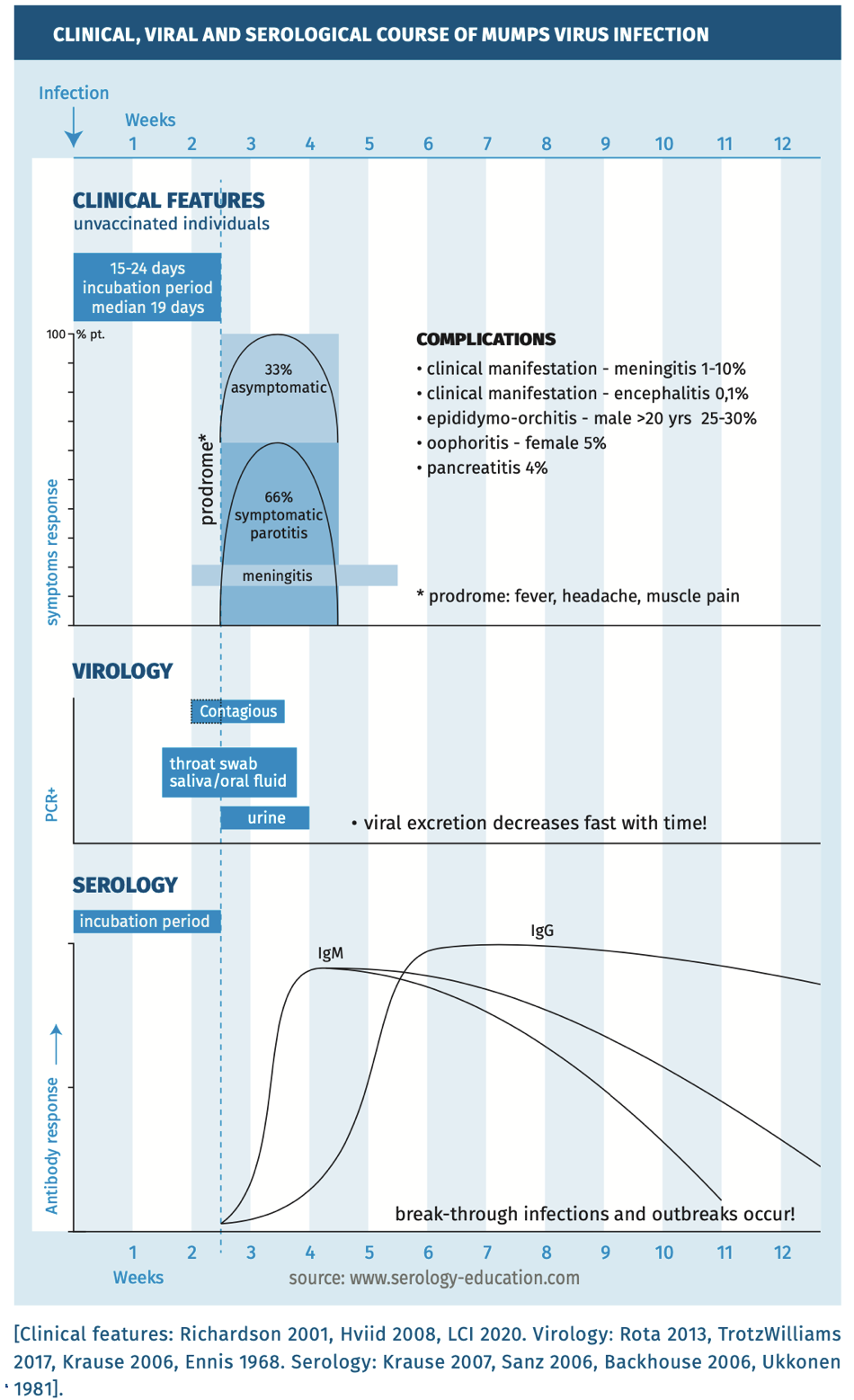
The incubation period is 15 to 24 days (median, 19 days) [Hviid 2008]. Mumps is acquired through the respiratory route by inhalation or oral contact with infected respiratory droplets or secretions [Rubin 2015]. Infection can remain localized to the respiratory tract but transient viraemia is probably frequent, occurring late in the incubation period, resulting in viral spread to organs. Infected mononuclear cells can also contribute to systemic viral spread [Hviid 2008].
Approximately one-third to one-half of infections are asymptomatic or result in a self-limiting disease with mild respiratory symptoms, sometimes accompanied by fever [Rubin 2015]. In a typical presentation symptoms include headache, malaise, myalgia, anorexia and fever [Rubin 2015, Lam 2020]. After the prodromal phase, the disease is characterized by painful swelling of the parotid glands (parotitis), but numerous other tissues and organs can be involved, resulting in inflammatory reactions in multiple organs, including encephalitis, meningitis, orchitis, myocarditis, pancreatitis and nephritis [Rubin 2015]. Most commonly, unilateral orchitis is seen in men (10-30%), accompanied by epididymitis and fever, which usually resolves within a week and only rarely causes reduced fertility.
In post pubertal women, mastitis and oophoritis (manifesting as pelvic pain and only rarely causing infertility or premature menopause) occurs in 5–10% of mumps cases. [Rubin 2015]. Most cases recover within a few weeks. Mumps is a vaccine preventable disease. The vaccine used is a live attenuated vaccine, which is combined with measles and rubella. Post vaccination breakthrough infections of mumps most often occur in adolescents and (young) adults and are usually associated with milder disease [Gouma 2016].
Complications
The major complications in mumps are meningitis and encephalitis. The virus is highly neurotropic, with laboratory evidence of central nervous system (CNS) infection in approximately half of the cases (most without neurological symptoms). Symptomatic CNS infection is less common, but significant. Meningitis occurs in approximately 5–10% of cases and encephalitis in <0.5%. Long-term sequelae, such as paralysis, seizures, cranial nerve palsies, hydrocephalus and deafness, may occur. Deafness has been reported in approximately 4% of mumps cases and was the most frequent cause of acquired unilateral sensorineural hearing loss in children. Hearing loss is typically transient, but can be permanent. The case fatality rate is 1.6–3.8/10,000, with most fatalities occurring in persons with encephalitis [Rubin 2015].
Although transplacental transmission is reported, mumps virus does not appear to cause congenital malformations [Rubin 2015, White 2012].
Epidemiology
Mumps cases are mainly seen in late winter and early spring but do occur throughout the year [White 2012]. The epidemiology is largely determined by the respiratory mode of transmission through contact with droplets of saliva or mucus from mouth, nose or throat of infected persons [Rubin 2015, White 2012]. It is a moderately to highly contagious infection, with an estimated basic reproductive rate of 4.4 (varying from 3.3 to 10.3). Transmission may also occur from persons with asymptomatic infections and vaccinated individuals [Fanoy 2011] highlighting the challenges in preventing virus transmission [Rubin 2015, Lam 2020]. It is assumed that the infectious period starts 1 to 2 days before onset of clinical symptoms and continues for 5 days after resolving of symptoms [Hviid 2008, Lam 2020].
The current estimated burden of mumps disease is highly dependent upon the vaccination coverage. There is no recent accurate estimate of the global mumps disease burden in the published literature. In contrast to measles and rubella, in recent years several large outbreaks of mumps have been reported in vaccinated individuals.
In a highly vaccinated population there is a low prevalence of disease. Due to waning immunity, currently, most mumps infections occur in (twice) vaccinated adolescents and (young) adults. Maternal antibodies may protect neonates in the first months, and in children of naturally immune women antibodies persist for a longer period compared to children of vaccinated women [Leuridan 2012].
Diagnostic testing
The clinical, viral and serological course are depicted in figure 1.
Figure 1.
Techniques
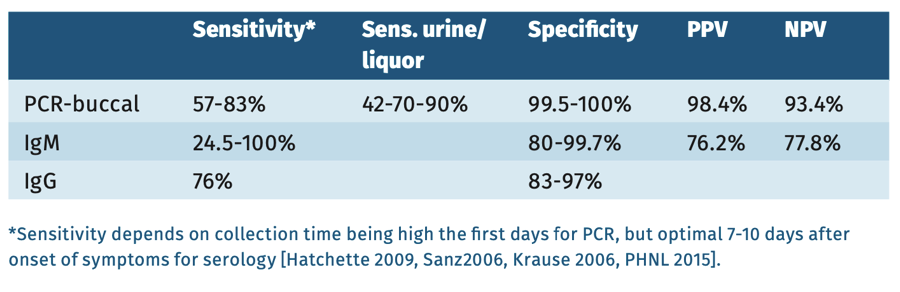
Molecular testing: detection of mumps virus RNA in clinical samples such as throat swab, oral fluid, urine, using mumps RNA-PCR [Krause 2006, Cooley 2015, Hatchette 2009] is recommended during the first week after onset of clinical signs [Mankertz 2008]. PCR is the preferred metod in all vaccinated individuals.
Serology: EIA IgM capture or IgG detection of a significant increase in the mumps virus IgG antibody titer in paired (acute and convalescent) serum samples [Borgmann 2014, Gouma 2014]. Sample collection for serology should be performed ≥3 days after onset of disease [Rota 2009]. In serum samples collected <3 days after onset of disease, mumps-virus specific IgM is often negative. IgM peaks 7 days after onset, and decreases after 10 weeks. Following IgM, IgG quickly rises, peaking at three weeks after onset remaining high for 2 months, before slowly declining (to 25% in 20 years).
Practical use of serology
Screening
IgG antibodies can be used for screening for immunity and for epidemiological research.
Suspected infection in immunocompetent patients
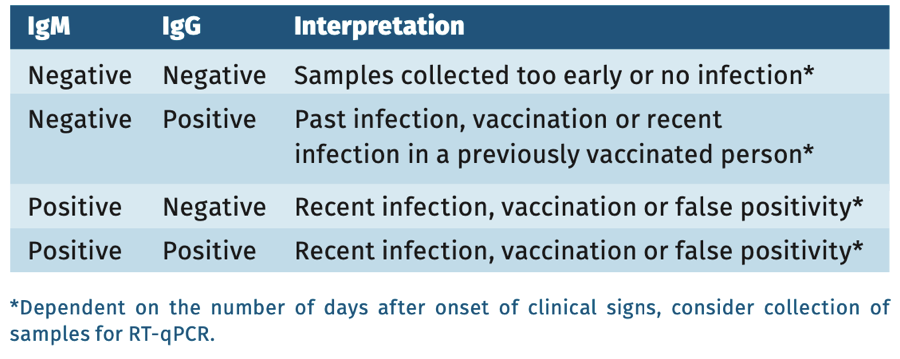
In nonvaccinated individuals with clinical signs of mumps virus infection, IgM EIA can be performed from 3-5 days after onset of symptoms. If IgM testing is negative, a mumps PCR or repeat serology testing after 1-2 weeks with IgM and IgG (paired) can be performed.*’ **
* In a highly vaccinated population, the laboratory case definition for infected individuals states that IgM is only suggestive of infection, and should always be confirmed by one or more definitive criteria: isolation of the virus or a PCR on clinical material or a fourfold rise in IgG titer.
** In outbreak settings, and especially in young children, late after exposure oral fluid samples (or saliva samples) can be used for detection of mumps virus-specific IgM, although with somewhat lower sensitivity compared to mumps virus-specific IgM on serum [Warrener 2006].
Suspected infection in immunocompromised patients
Infection should preferably be proven by mumps PCR or second best through a positive IgM or rise in IgG titer in paired serum samples.
Interpretation of serology
In symptomatic but vaccinated patients a single serum sample can not provide definite proof of an infection. For diagnostic purpose always collect sample(s) for PCR within 1 week of onset symptoms (see table 1) or use paired serum samples whatever the result of the first sample to prove a fourfold/significant titer rise.
Table 1.
Sensitivity and specificity
In general, commercially available mumps virus-specific IgM EIA have good specificity. The sensitivity of commercially available mumps virus specific IgM kits varies greatly though, with a better sensitivity for capture assays [Mankertz 2015, Rota 2013, Krause 2007, Haywood 2014]. In a highly vaccinated population prevalence of disease is very low and the predictive values of tests are poor, always needing confirmation (with PCR) [Warrener 2006].
Table 2.
pitfalls
- See the general chapter “pitfalls”.
References
- Backhouse JL, Gidding HF, McIntyre PB et al. Evaluation of two enzyme immunoassays for detection of immunoglobulin G antibodies to mumps virus. Clin Vaccine Immunol 2006;13:764-67.
- Borgmann S, Schwab F, Santibanez S et al. Mumps virus infection in vaccinated patients can be detected by an increase in specific IgG antibodies to high titres: a retrospective study. Epidemiol Infect 2014;142(11):2388-96.
- Cooley L PHLN. Mumps Laboratory case definition-version 1.0 2015.
- Ennis FA, Jackson D. Isolation of virus during the incubation period of mumps infection. J Pediatr 1968;72:536-37.
- Fanoy EB, Cremer J, Ferreira JA et al. Transmission on mumps virus from mumps vaccinated individuals to close contacts. Vaccine 2011;29:9551-9556.
- Gouma S, Schurink- Van ‘t Klooster TM, De Melker HE et al. Mumps serum antibody levels before and after an outbreak to assess infection and immunity in vaccinated students. Open Forum Inf Dis 2014;1(3):ofu101.
- Hatchette TF, Davidson R, Clay S et al. Laboratory diagnosis of mumps in a partially immunized population 2009;20(4):157-162.
- Haywood, B Patel, M, Hurday S et al. Comparison of automated chemiluminescence immunoassays with capture enzyme immunoassays for the detection of measles and mumps IgM antibodies in serum. J Virol Methods 2014;196:15-7.
- Hviid A, Rubin S, Muhlemann K. Mumps. Lancet 2008;371(9616):932-44.
- ICTV. Virus Taxonomy: 2019 Release. 2020; Available from: https://talk. ictvonline.org/taxonomy/.
- Krause CH, Molyneaux DO, Ho-Yen P et al. Comparison of mumps-IgM ELISAs in acute infection. J Clin Virol 2007;38(2):153-6.
- Krause CH, Eastick K, Ogilvcie MM. Real-time PCR for mumps diagnosis on clinical specimens-comparison with results of conventional methods of virus detection and nested PCR. J Clin Virol 2006;37:184-89.
- Lam E, Rosen JB, Zucker JR. Mumps: an Update on Outbreaks, Vaccine Efficacy, and Genomic Diversity. Clin Microbiol Rev 2020;33(2).
- LCI. Bof Richtlijn RIVM 2020.
- Leuridan E, Goeyvaerts N, Hens V et al. Maternal mumps antibodies in a cohort of children up to the age of 1 year. Eur J Pediatr 2012;171(8):1167-73.
- L’Huillier AG, Eshaghi A, Racey CS et al. Laboratory testing and phylogenetic analysis during a mumps outbreak in Ontario. Canada. Virol J 2018;15(1):98.
- Mankertz A, Beutel U, Schmidt FJ et al. Laboratory-based investigation of suspected mumps cases submitted to the German National Reference Centre for Measles, Mumps, and Rubella, 2008 to 2013. Int J Med Microbiol 2015;305(7):61926.
- Maillet M, Bouvat E, Robert N et al. Mumps outbreak and laboratory diagnosis. J Clin Virol, 2015;62:14-19.
- Nunn A, Masud S, Krajden M et al. Diagnostic Yield of Laboratory Methods and Value of Viral Genotyping during an Outbreak of Mumps in a Partially Vaccinated Population in British Columbia, Canada. J Clin Micr 2018;56(5):
e01954-17. - Richardson M, Elliman D, Maguire H et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in school and preschools. Paediatr Infect Dis J 2001;20:380-91.
- Rota JS, Rosen JB, Doll MK, et al. Comparison of the sensitivity of laboratory diagnostic methods from a well-characterized outbreak of mumps in New York city in 2009. Clin Vaccine Immunol, 2013;20(3):391-6.
- Rubin S, Eckhaus M, Rennick LJ et al. Molecular biology, pathogenesis and pathology of mumps virus. J Pathol 2015;235(2):242-52.
- Sanz JC, Mosquera MM, Echevarria JE et al. Sensitivity and specificity of immunoglobulin G titer for the diagnosis of mumps virus in infected patients depending on vaccination status. APMIS 2006;114:788-94.
- Trotz Williams LA, Mercer HJ, Paphitis K et al. Challenges in interpretation of diagnostic test results in a mumps outbreak in a highly vaccinated population. Clin Vaccin Immunol 2020;24(2):1-7.
- Ukkonen P. Mumps specific immunoglobulines M and G antibodies in natural mumps infection as measured by enzyme linked immunoassay. J Med Vir 1981;8(2):131-142.
- Waaijenborg S, Hahne SJ, Mollema L et al. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. J Infect Dis 2013;208(1):10-6.
- Warrener L, Samuel D. Evaluation of a commercial assay for the detection of mumps specific IgM antibodies in oral fluid and serum specimens. J Clin Virol, 2006; 35(2):130-4.
- White SJ, Boldt KL, Holditch SJ et al. Measles, mumps and rubella. Clin Obstet Gynecol 2012;55(2):550-9.