Keyword(s)
Rubella virus, German measles, three-day measles, Congenital rubella syndrome
Download this article 
Rubella virus index
Index of pathogens
[Please click on the initial letter of the pathogen or simply scroll down the list!]
Bodewes r, Voordouw B, Petit p
The microorganism and its clinical presentation
Rubella is a contagious respiratory viral infection caused by the rubellavirus. Rubellavirus is an enveloped single stranded positive sense RNA virus belonging to the Matonaviridae family [ICTV 2020]. It was the sole member of the Rubivirus genus, but recently two closely related viruses were discovered in mammals [Bennett 2020].
Up to 50% of infections are asymptomatic or subclinical, especially in children [Leung 2019]. The incubation time ranges between 12 and 23 days (average 1416 days) [Leung 2019, WHO 2021, Lanzieri 2020]. In symptomatic disease, also called “German measles”, the initial symptoms following the incubation period typically include lowgrade fever, malaise, lymphadenopathy and an upper respiratory infection for 15 days followed by a brief appearance of a rash [Leung 2019, White 2012]. Forchheimer spots (petechiae on the soft palate) may precede or accompany the rash. The rash is mild and maculopapular, beginning on the face and extending downwards. It occurs approximately 14 to 17 days after exposure and typically lasts three days. Occasionally the rash is accompanied by pruritus [White 2012, Lanzieri 2020]. Rubella is generally a mild, selflimiting infectious disease [Leung 2019]. Enlarged postauricular and suboccipital lymph nodes, which precede the rash, are characteristic of rubella and last for 5–8 days. (https://www.ecdc. europa.eu/en/rubella/factsheet). Definite diagnosis on clinical features is unreliable and needs confirmation.
The vaccine used is a live attenuated vaccine that also can cause a rubellalike disease but is not contagious! Neonatal protection by maternal antibodies is 45 months.
Complications
The real threat arises when acute rubella infection occurs in pregnancy, particularly in the first trimester when it can infect the fetus, which may lead to miscarriage (20%), intrauterine fetal death, premature labour, intrauterine growth retardation, or congenital rubella syndrome (CRS) [Leung 2019, Voordouw 2019, Lambert 2015] producing anomalies in the developing fetus. Cataract, congenital heart defect, and sensorineural deafness are the classic triad of congenital rubella syndrome [Leung 2019].
In postnatal infections rubella frequently leads to joint symptoms including arthralgia or arthritis in women (up to 70%) along with conjunctivitis, but these are rare in males and children [White 2012, Lanzieri 2020]. Haemorrhagic manifestations (mainly thrombocytopenic purpura) occur in approximately 1 per 3000 cases. Effects may last from days to months, and most patients recover. Encephalitis with an estimated incidence of 1 in 6000 cases is reported and may be fatal. Additional rare complications include granulomas in persons with primary immune deficiencies, orchitis, neuritis, and a late syndrome of progressive panencephalitis [Lanzieri 2020].
Epidemiology
Rubella is a moderately contagious infection, with an estimated basic reproductive rate of 7-8 [LCI 2015]. Postnatal transmission of the virus
is primarily by inhalation of droplets or direct contact with nasopharyngeal secretions from infected persons [White 2012]. The infectious period starts one week before onset of clinical symptoms and continues for one week after development of the characteristic rubella rash [Leung 2019]. Infants with congenital rubella syndrome (CRS) shed large quantities of the rubella virus from their bodily secretions for more than a year postpartum and are highly contagious [White 2012]. Transmission may also occur from persons with asymptomatic or subclinical infection [White 2012], highlighting the challenges of preventing virus transmission.
Rubella affects people globally. In the absence of vaccination, the mean age of rubella infection is 5–9 years with annual seasonal outbreaks usually occurring in the spring. Large epidemics occur every 3–8 years [Lambert 2015]. The last small rubella outbreak in The Netherlands occurred in 2013 with 54 reported cases. Vaccination programs have led to a shift in demography to individuals of childbearing age, which increases the risk of CRS [White 2012]. Antibodies against rubella virus after natural infection persist longer than antibodies mounted after vaccination [Waaijenborg 2013]. Epidemic outbreaks continue to occur, particularly in settings with partial vaccination strategies [Lambert 2015, Abrams 2016], for example the ‘Bible Belt’ in The Netherlands. Especially with inadequate vaccination coverage rubella remains endemic world wide [White 2012].
Diagnostic testing
The clinical, viral and serological course are depicted in figure 1.
Figure 1.
Techniques
- Molecular: Rubella PCR to detect RubellaRNA in throat swab, saliva or urine. Rubella causes a lowlevel, shortlived viraemia which is difficult to detect [Abernathy 2009].*
- Serology: EIA IgM capture has the highest specificity to prove recent infection [Wandinger 2011, Hubschen 2017]. Test >4 days after onset of rash [Abernathy 2009, Kurata 2019]. All commercial immunoassays are calibrated against a WHO international standard, but no other criteria are tested nor standardized [ValoupFellous 2018]. Results can therefore not be compared.
- An IgG EIA with standard reference for intensity of response will confirm specificity or in paired serum samples (one acute and one
2 weeks later) showing a fourfold or substantial increase in antibodies to prove infection. IgG will be positive >4 days after onset rash, increasing rapidly and being high for 23 months then declining slowly but remaining positive for life.** - IgG-avidity: a commercially available test that proves or exclude a recent infection in pregnancy or in doubtful cases and in absence of a second serum [Böttiger 1997]. This can be requested at the RIVM.
*Virus culture: as the occasion arises virus can be cultured at the RIVM.
**IgG-E2: E2 envelope protein in rubella virus (RV) occurs 3-4 months after onset of infection. Can be used in pregnancy to exclude recent infection (used in several European countries). A Commercial EIA is available.
Practical use of serology
Screening
Immunity after vaccination or past infection is do with a specific IgG test, validated with a reference. Level of antibody titers are not indicative of protective immunity. Results should always be confirmed in case of Screening complications like CRS. IgM can be positive after vaccination and can stay positive for >1 year [Thomas 1992, Banatvala 1985]. In these cases of possible recent infection confirmation is deemed necessary and infection can be proven by PCRRV or IgGavidity testing on a single serum sample or with a validated IgG EIA on a paired serum sample.
Suspected infection in immunocompetent patients including pregnant women
This is proven by a rubella specific IgM which starts to be positive >4 days and remaining high for 13 months after the onset of the rash. Serum should be collected 530 days after development of rash. If collected within 5 days after the onset of rash or start of symptoms rubella PCR or a paired serum with at least 2 weeks in between is recommended for laboratory diagnosis of a recent infection [WHO 2018, Kurata 2019, Abernathy 2009, Cordoba 1991, Uchino 2020]. Avidity testing in pregnancy can be done to determine the start and duration of infection.
A suspected infection in immunocompromised patients
This needs a RubellaPCRRNA to prove infection. If immunoglobulins are present tests similar to immunocompetent patients can be performed.
Congenital Rubella Syndrome cases
PCR on saliva at birth, postnatal IgM and PCR on urine with followup serology is necessary.
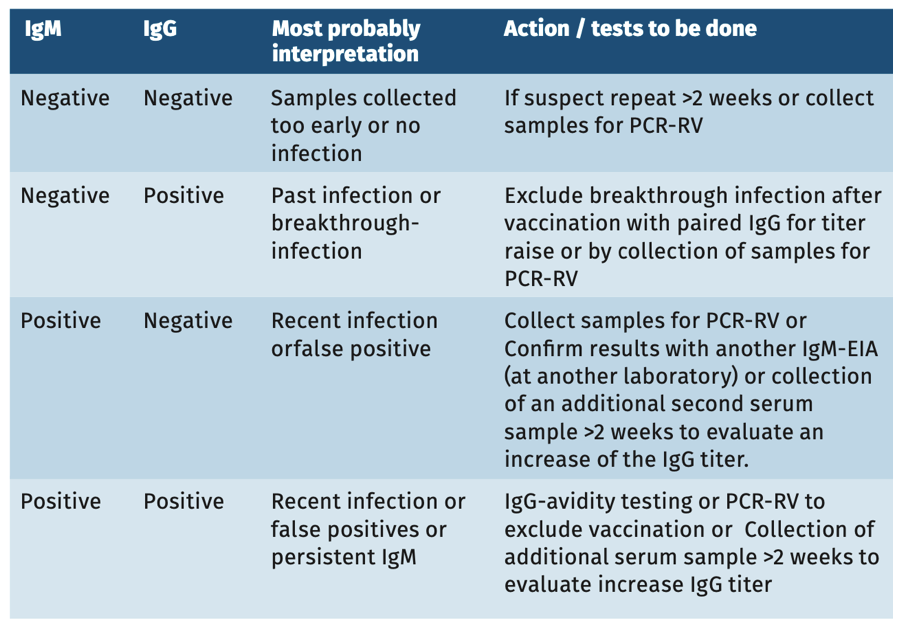
Interpretation of serology
In symptomatic but vaccinated patients a single serum sample can not provide definite proof of an infection. For diagnostic purpose always collect sample(s) for PCR within 1 week after the onset of symptoms (see table 1) or use paired serum samples whatever the result of the first sample to prove a fourfold/significant titer rise.
TABLE 1. SEROLOGY IN AN IMMUNOCOMPETENT PERSON WITH CLINICAL SIGNS OF RV INFECTION
TABLE 2. SEROLOGY IN A NEWBORN CHILD (<6 MONTHS) SUSPECTED OF CRS
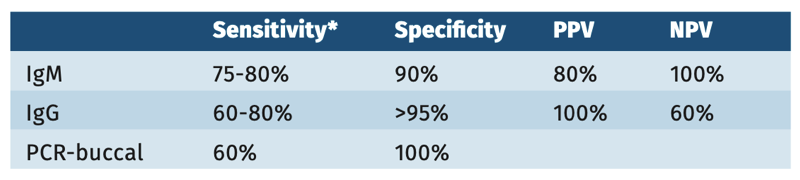
Sensitivity and specificity
Specificity of the commercially available rubella IgM kits in general is high, but sensitivities depend very much on people, time after onset, cutoffs used and format of antigens [WHO 2018, Echevarria 1985]. WHO has collected sera from all over the world and assessed the performance of EIA’s against PCR confirmed cases. The results of these comparison studies will be presented soon [PubMed (nih.gov) 2021].
Table 3.
Pitfalls
- See for pitfalls [Best 2002] and cross reactions [Lefrere 1987] the general chapter “Pitfalls in serology”.
- Rubella has no specific clinical or pathognomonic presence.
References
- Abernathy, E., Cabezas C, Sun H et al., Confirmation of rubella within 4 days of rash onset: comparison of rubella virus RNA detection in oral fluid with immunoglobulin M detection in serum or oral fluid. J Clin Microbiol, 2009. 47(1): p. 182-8.
- Abrams S, Kourkoum E, Sable M et al. Inferring rubella outbreak risk from seroprevalence data in Belgium. Vaccine 2016;34:618792.
- Banatvala, J.E., Best JM, O’Shea S et al., Persistence of rubella antibodies after vaccination: detection after experimental challenge. Rev Infect Dis, 1985. 7 Suppl 1: p. S86-90.
- Bennett AJ, Paskey AC, Ebinger A et al. Relatives of rubella virus in diverse mammals. Nature 2020;586:424428.
- Best, J.M., O’Shea S, Tipples G et al., Interpretation of rubella serology in pregnancy--pitfalls and problems. BMJ, 2002. 325(7356): p. 147-8.
- Böttiger B, Jensen IP. Maturation of rubella IgG avidity over time after acute rubella infection. Clin Diagn Virol, 1997. 8(2): p. 105-11.
- Cordoba, P., Nates S, Mahoney J et al., Kinetics of rubella-specific IgM antibody response in postnatal rubella infection. J Virol Methods, 1991. 34(1): p. 37-43.
- Davidkin I, Jokinen S, Broman M et al. Persistence of measles, mumps and rubella antibodies in an MMRvaccinated cohort: a 20 year followup. J Infect Dis 2008;197:95056.
- Dimech W, Mulders MN. A 16-year review of seroprevalence studies on measles and rubella. Vaccin 2016;34(35):4110-4118.
- Echevarria C, Fernandez MV, Echevarria JM et al. Pregnant women in contact with rubella. J Infect 1985;11:802.
- Hubschen JM, Bork SM, Brown K.E. et al. Challenges of measles and rubella laboratory diagnostic in the era of elimination. Inf. 2017;23:51115.
- ICTV 2020 Virus Taxonomy: 2019 Release. https://talk.ictvonline.org/taxonomy/. Accessed.
- Kurata, T., Uchino K, Hotta C et al., Clinical value of enzyme immunoassay that detects rubella-specific immunoglobulin M immediately after disease onset. Microbiol Immunol, 2019. 63(1): p. 32-35.
- Lambert N, Strebel P, Orenstein W et al. Rubella. Lancet 2015; 385:2297307.
- Lanzieri T, Haber P, Icenogle JP. Updated December 2020. Rubella. https://www. cdc.gov/vaccines/pubs/pinkbook/rubella.html. Accessed.
- Leung AKC, Hon KL, Leong KF. Rubella (German measles) revisited. Hong Kong Med J 2019;25(2):134141.
- Lefrere JJ, Bricout F, Bertrand Y et al. Human Parvovirus and Rubella cross0reactions in specific IgM tests. The Lancet 1987;329(8523):501987. LCI. Rodehond Richtlijn RIVM 2015:112.
- O’Shea S, Woodward S, Best JM et al. Rubella vaccination: persistence of antibodies for 1021 years. Lancet 1988;2:909.
- PubMed (nih.gov) 2021. Evaluation of diagnostic accuracy of eight commercial assays for the detection of rubella specific IgM antibodies.
- Thomas, H.I., Morgan-Capner P, Roberts A et al., Persistent rubella-specific IgM reactivity in the absence of recent primary rubella and rubella reinfection. J Med Virol, 1992. 36(3): p. 188-92.
- Uchino, K., Miyoshi T, Mori Y et al., Comparison of virological and serological methods for laboratory confirmation of rubella. J Clin Virol, 2020. 123: p. 104257.
- Voordouw B, Rockx B, Jaenisch T et al. Performance of Zika Assays in the Context of Toxoplasma gondii, Parvovirus B19, Rubella Virus, and Cytomegalovirus (TORCH) Diagnostic Assays. Clin Microbiol Rev 2019;33(1).
- Vauloup-Fellous C, Grangeot-Keros L. Humoral immune response after primary rubella virus infection and after vaccination. Clin Vaccine Immunol, 2007. 14(5): p. 644-7.
- Valoup-Fellous C. Standardization of rubella immunoassays. J Clin Virol 2018;102:34-38
- Waaijenborg S, Hahne SJ, Mollema L et al. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. J Inf Dis 2013;208(1):106.
- Wandinger KP, Saschenbecker S, Steinhagen K et al. Diagnosis of recent primary rubella virus infections: significance of glycoprotein-based IgM serology, IgG avidity and immunoblot analysis. J Vir Methods 2011;174:859.
- White SJ, Boldt KL, Holditch SJ et al. Measles, mumps, and rubella. Clin Obstet Gynecol 2012;55:5509.
- WHO. Site accessed Jan 25, 2021. Rubella. http://www.emro.who.int/ healthtopics/rubella/index.html. Accessed.
- WHO. Manual for the Laboratorybased Surveillance of Measles, Rubella, and Congenital Rubella Syndrome, Third edition 2018.https://www.who.int/ immunization/monitoring_surveillance/burden/laboratory/manual_ section7.3/en/. Accessed.
- Wilson KM, Di Camillo C, Doughty I et al. Humoral immune response to primary rubella infection. Clin Vaccine Immunol 2006;13:38086.
Keywords: Rubella virus, German measles, three-day measles, Congenital rubella syndrome