Hepatitis E virus index
Index of pathogens
[Please click on the initial letter of the pathogen or simply scroll down the list!]
Ang W, Petit P
The microorganism and its clinical presentation
Hepatitis E virus (HEV) is a single-stranded RNA virus, classified in the family of Hepeviruses. HEV comprises of multiple genotypes (gt).
Gt 1 - gt4 and gt7 are found in humans. Gt 1 and gt2 seem to be restricted to humans. The others have been detected in animals, including pigs (gt 3, gt 4) and camelids (gt 7, gt 8). Gt 3 is the only genotype that is prevalent worldwide, whereas the others are limited to specific regions.
Transmission is mainly human-to-human and zoonotic.
The incubation time is 2-8 (average 4) weeks. Most cases are asymptomatic. Clinical symptoms range from mild to severe acute hepatitis leading to liver failure in a minority of patients, predominantly middle aged men. In children, the disease is mostly asymptomatic or mild
[Al-Sadeq 2018, Kamar 2014, LCI 2019]; symptoms increase with age.
A vaccine against HEV infection is licensed only in China.
Complications
HEV infections in immunocompromised patients may lead to chronic HEV infection, defined as persistence of HEV RNA in blood for a least six months. Rare complications are liver failure and neurological diseases (Guillain-Barré syndrome or neuralgic amyotrophy). Pregnant women may have an increased risk of severe disease and death when infected by gt 1 and gt 2 [Kamar 2014].
Epidemiology
Gt 1 and gt 2 are mainly present in India, Pakistan and Bangladesh, and infection with these genotypes is considered to be travel-associated (human to human: faeco-oral) in other countries. Gt 3 and to a lesser extent gt 4 are found as a cause of food-borne infection (faecally contaminated and inadequately heated) in many high-income countries. Symptomatic gt 3 patients are predominantly males >50 years, with pre-existing liver disease or persons with an impaired immune system.
Transmission by contaminated blood products and vertical transmission has been documented [LCI 2019, Zaaijer 2015, Bi 2020].
The seroprevalence of HEV depends heavily on the selected serological assay to detect IgG antibodies (see pitfalls). Although the seroprevalence in industrialised countries ranges from <1% (Norway) to >20% (France), there are regional differences observed within these countries. The average seroprevalence ranges from 1-10% in high-income countries, to 20% in sub-Saharan Africa to 2-70% in Asia. There is a potential birth cohort effect resulting in higher seroprevalence rates in older patients. Chronic infection occurs in immunocompromised patients and can be difficult to treat. Reinfection seems possible based on vaccination studies, cohort studies and case reports but is rare [Abravanel 2014, Baylis 2015].
Diagnostic testing
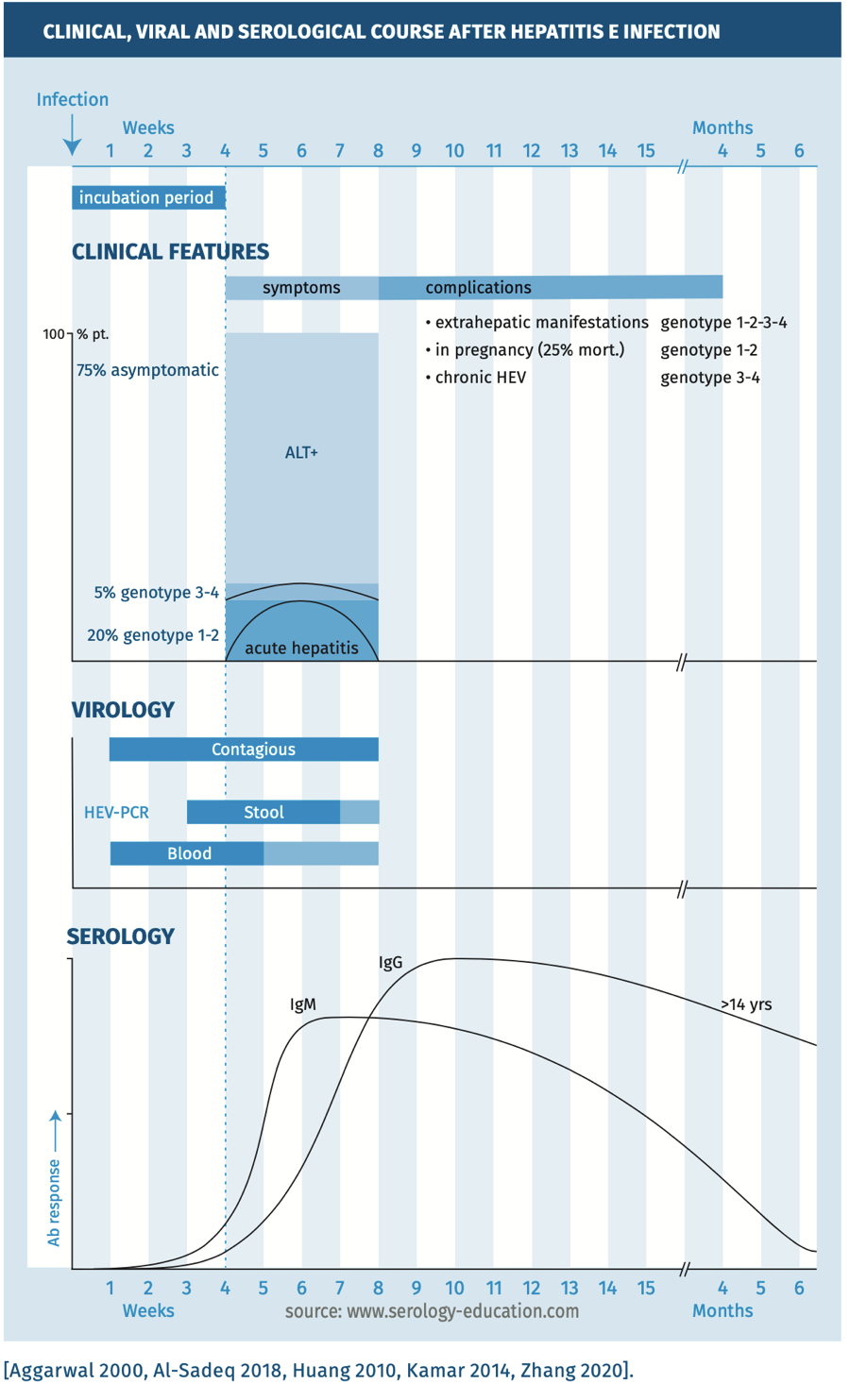
The clinical, viral and serological course are depicted in figure 1.
Figure 1.
Techniques
- Molecular: HEV-PCR on blood, faeces and body fluids (prolonged secretion of HEV RNA in immunocompromised patients >3 months).
- Serological: EIAs IgM and IgG to test for anti-HEV antibodies. Tests use recombinant genotype 1 antigen which reacts with all genotypes and does not show cross-reactions with other pathogens. There is a WHO standard reference test for IgG to evaluate analytical sensitivity [Ferguson 2001]. As in most tests, IgM is subject to false positivity by EBV and CMV (see general chapter pitfalls) [Hyams 2014]. Other tests are not used in the Netherlands* **.
- Immunoblot: used for confirmation of specificity [Herremans 2007] if PCR is not available and in cases with positive IgM and negative PCR.
* IgG tests also used for avidity testing to estimate the onset of infection [Bigaillon 2010].
** Antigen tests: to detect HEV capsid positive 2 weeks after exposure, remaining positive for 4-5 weeks.
Practical use of serology
Screening
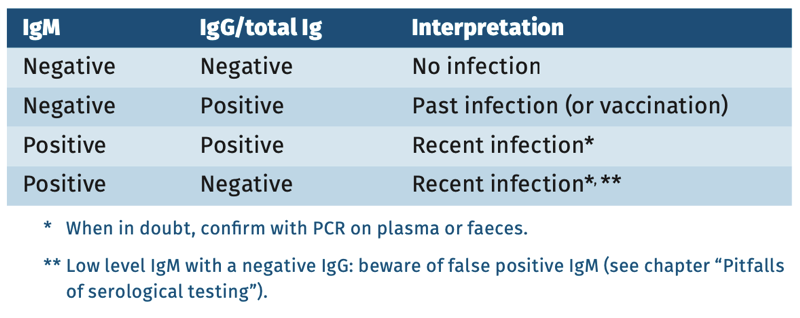
Testing for past infection and vaccination: IgG anti-HEV with WHO reference [Bendall 2010]. Screening is not generally recommended because the presence of IgG does not indicate immunity against a new infection.
Suspected infection in immunocompetent child or adult
First choice is anti-HEV-IgM positive 4 weeks after exposure. Confirmation is carried out with HEV PCR. IgM and IgG anti-HEV can be performed on serum. HEV PCR on faeces or plasma is used to confirm results. Suspicious are laboratory confirmed hepatitis.
Suspected infection in immunocompromised child or adult
Perform HEV PCR on faeces or plasma because of delayed or absent IgM and IgG response.
Interpretation of serology
Table 1.
Sensitivity and specificity
Table 2.
In immunocompetent patients, IgM can most often be detected once clinical manifestations are present. Upon diagnostic testing, IgG is already detectable in most patients. IgM peaks around 6 weeks and gradually declines. IgG levels remain elevated over years and possibly lifelong.
The sensitivity and specificity of serological assays for HEV varies widely. In addition, the relative sensitivity for the different genotypes differs and manufacturers should evaluate their assay for suitability in detecting immune responses against emerging genotypes (such as gt 3) [Zhang 2020, Al-Sadeq 2018, Kar 2020].
In general, the sensitivity of IgM tests for detection of a recent infection with HEV ranges between 65-97%. The specificity ranges between 74100%. There is a WHO standard as reference only for IgG. Comparing results is not possible.
Another aspect of variation between different manufacturers is the long term IgG response. Some assays (e.g. Wantai) detect long-term antibody responses in much larger proportion of subjects than others (e.g. MP). The exact explanation for this phenomenon is not known, because the exact antigens and method of preparation are not disclosed for all assays and different cut-offs are used. When performing seroprevalence studies, this inter-test variation needs to be taken into account as it may lead to a significant bias between studies [Bendall 2010].
pitfalls
- A specific pitfall for HEV infections is the lack or delay of antibody responses in immunocompromised patients. In this group, PCR is the method of choice for detecting a recent or chronic HEV infection.
- Despite the fact that HEV is a known cause of acute hepatitis, testing for HEV is not always included in so-called Hepatitis panels when ordering tests. Doctors should check whether HEV testing is included in the algorithms for viral hepatitis.
- See general chapter “Pitfalls” for cross-reactions [Hyams 2014].
References
- Abravanel F, Lhomme SA, Chapuy-Regaud S et al. Hepatitis E virus reinfections in solid organ transplant recipients can evolve into chronic infections. The J of Inf Dis 2014;209(12):1900-1906.
- Aggarwal R, Kini D, Sofat S et al. Duration of viraemia and faecal viral excretion in acute hepatitis E. The Lancet 2000;356:1081-1082.
- Almeide Ponde de RA. The serological markers of acute infection with hepatitis A, B, C, D, E and G viruses revisited. Arch Virol 2017;162:3587-3602.
- Al-Sadeq DW, Majdalawieh AF, Mesleh AG et al. Laboratory challenges in the diagnosis of hepatitis E virus. J Med Micr 2018;67:466-480.
- Baylis SA, Crossan C, Corman VM et al. Unusual serological response to hepatitis E virus in plasmadonors consistent with reinfections. Vox Sanguinis 2015;109:406-409.
- Bendall R, Ellis V, Ijaz S et al. A comparison of two commercially available antiHEV IgG kits and a re-evaluation of anti-Hev IgG seroprevalence estimates. J Infect Dis 2010;82:799-805.
- Bi H, Yang R, Wu C et al. Hepatitis E virus and bloodtransfusion safety. Epid and Inf 2020;148:e158,1-9.
- Bigaillon C, Tesse S, Lagathu G et al. Use of hepatitis E IgG avidity for diagnosis of hepatitis E infection. J Virol Methods 2010;164: 127-130.
- Ferguson M, Walker D, Mast E et al. Report of a collaborative study to assess the suitability of a reference reagent for antibodies to hjepatitis E virus. Biologicals 2020;30:43-48.
- Herremans M, Bakker J, Duiser E et al. Use of serological assays for diagnosis of Hep E virus genotype 1 and 3 infections in a setting of low endemicity. Clin Vacc Immunol 2007;14(5):562-568.
- Huang S, Zhang X, Jiang H et al. Profile of acute infectious markers in sporadic hepatitis E. PLos One 2010;5(10):e13560.
- Hyams C, Mabayoje DA, Copping R et al. Serological cross-reactivity to CMV and EBV causes problems in the diagnosis of acute hepatitis E virus infection. J of Med Vir 2014;86:478-483.
- Kamar N, Dalton HR, Abravenel F et al. Hepatitis E virus infection. Clin Micr Reviews 2014;27(1):116-138.
- LCI Richtlijn Hepatitis E. RIVM 2019.
- Wu C, Wu X and Xia J. Hepatitis E virus infection during pregnancy. Virology Journal 2020;17(73):1-11.
- Zaaijer H. Hepatitis E. Nascholingscursus Infectieziekten 12, 13 en 14 maart 2015.
- Zhang Q, Zong X, Li D et al. Performance evaluation of different commercial serological kits for diagnosis of acute hepatitis E viral infection. Polish Journal of Microbiology 2020;69(2):217-222.
Keyword(s): Hepatitis E virus